08 Acid Base Principles
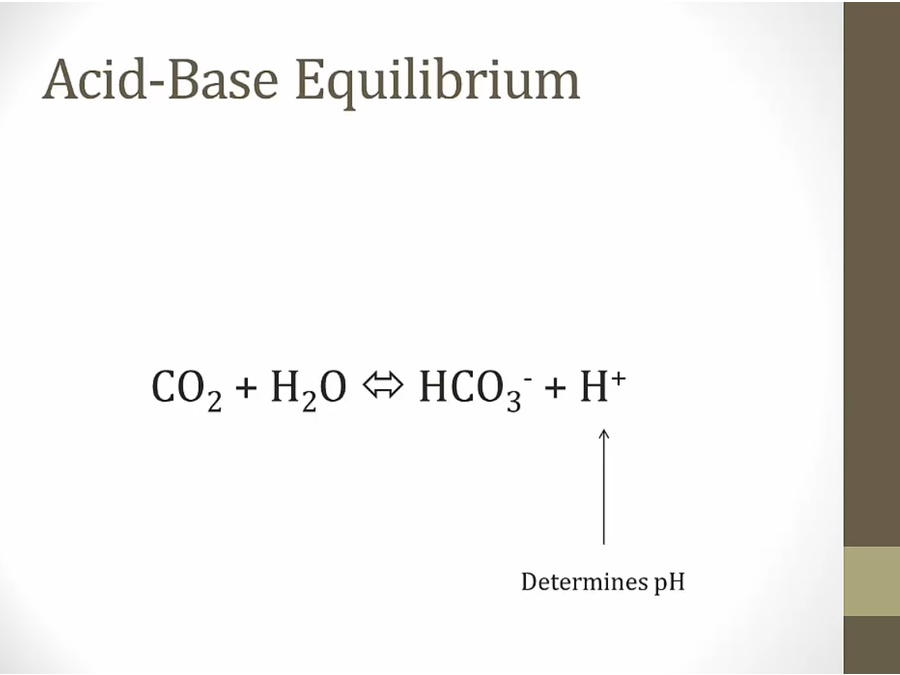
- Equilibrium constantly in plasma
- pH at set point
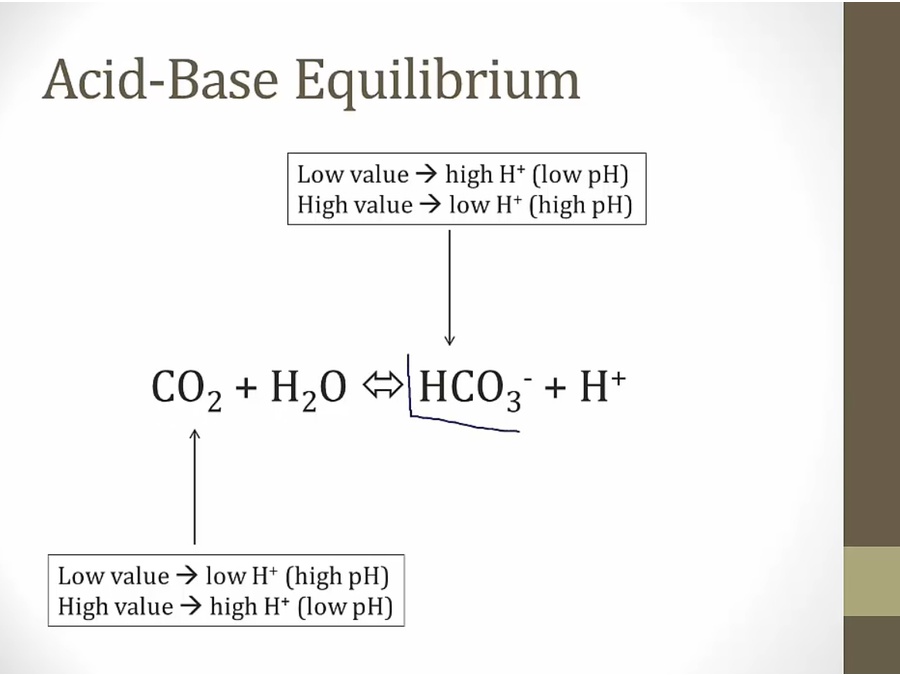
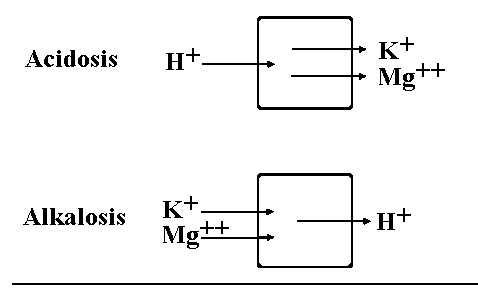
- low bicarb, drive equation right, make more H, acidic
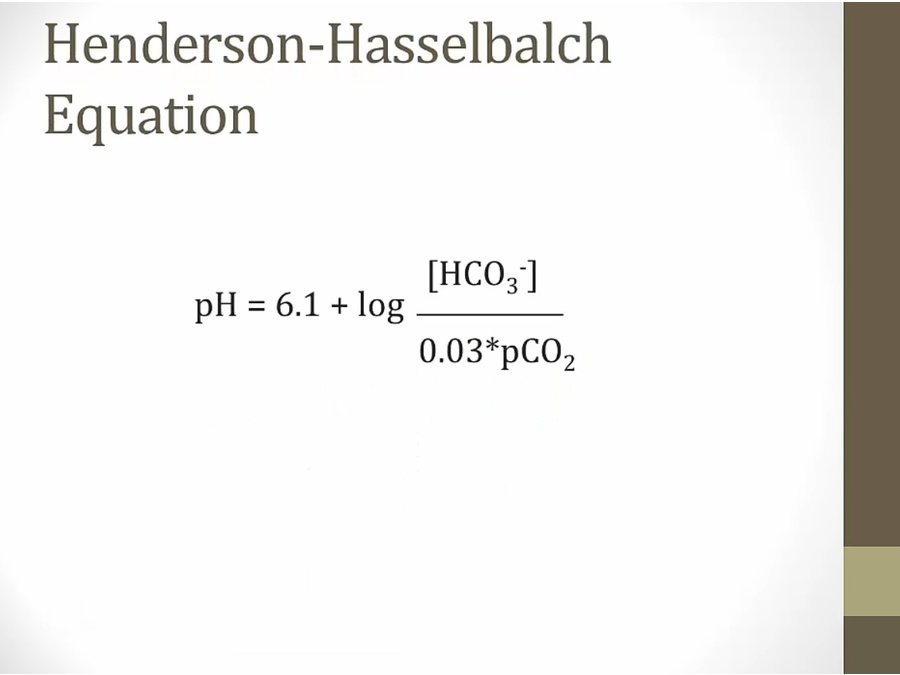
- don’t memorize
- bicarb up, pCO2 down, increase pH
- bicarb down, pCO2 up, lower pH
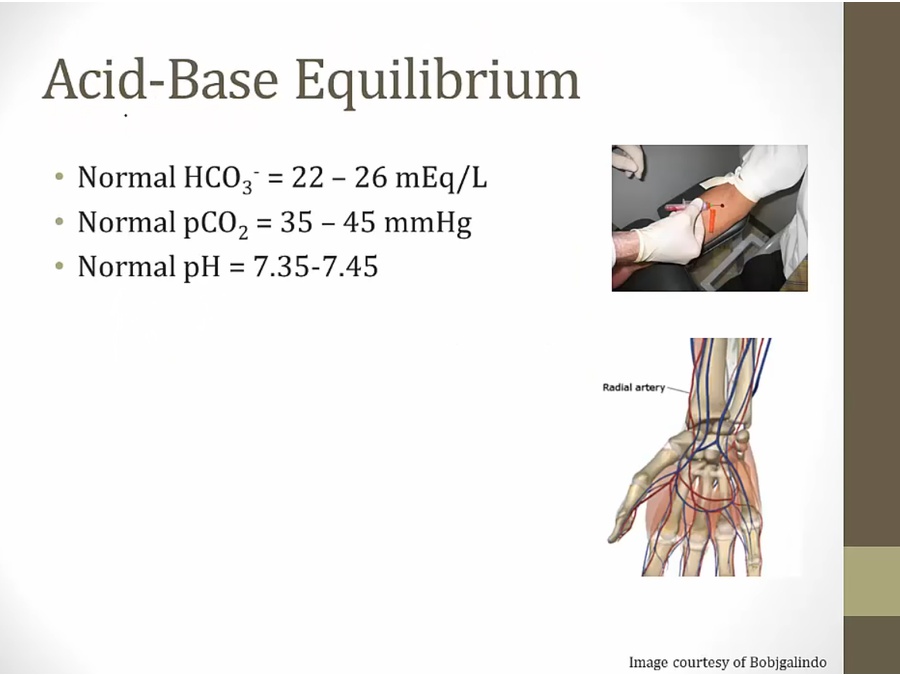
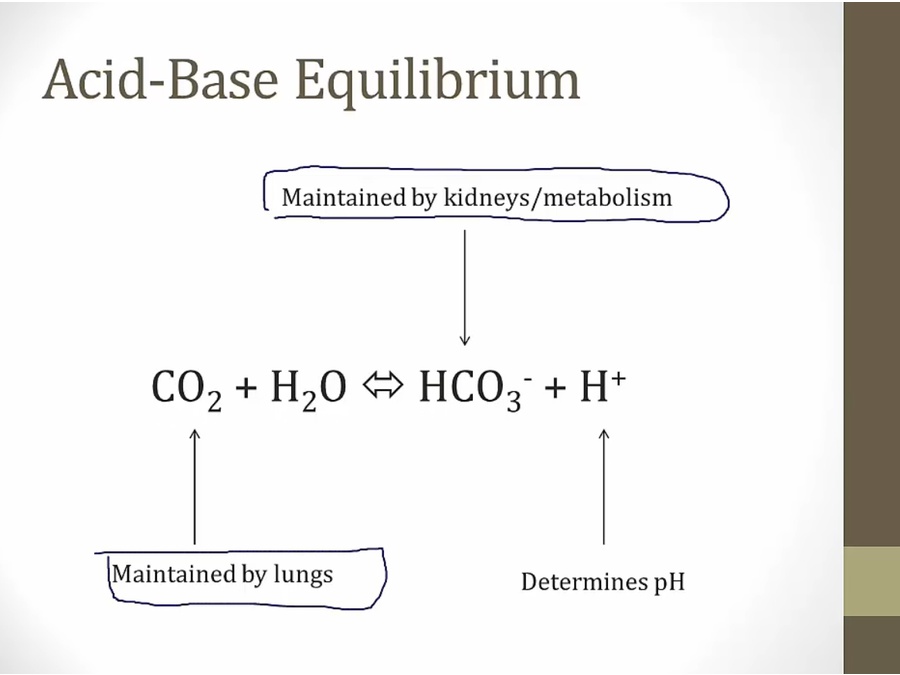
- sample taken in arterial sys, radial a, for accurate pCO2

Acidosis
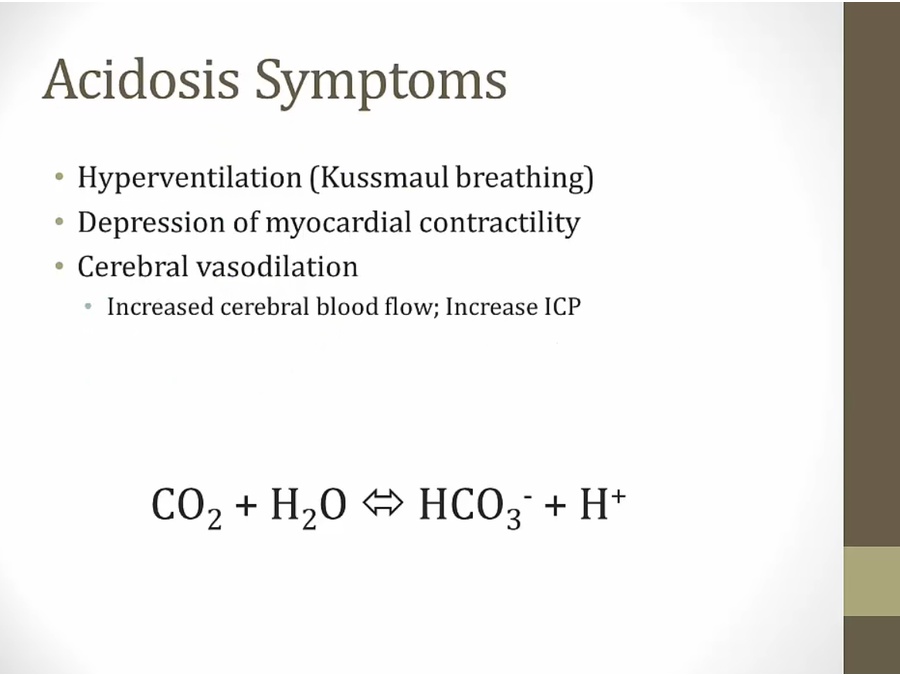
- blow CO2 off
- drop CO

- high CO2 = narcotic (people with pneumonia die painless death from high CO2 in older times)
Bohr effect:
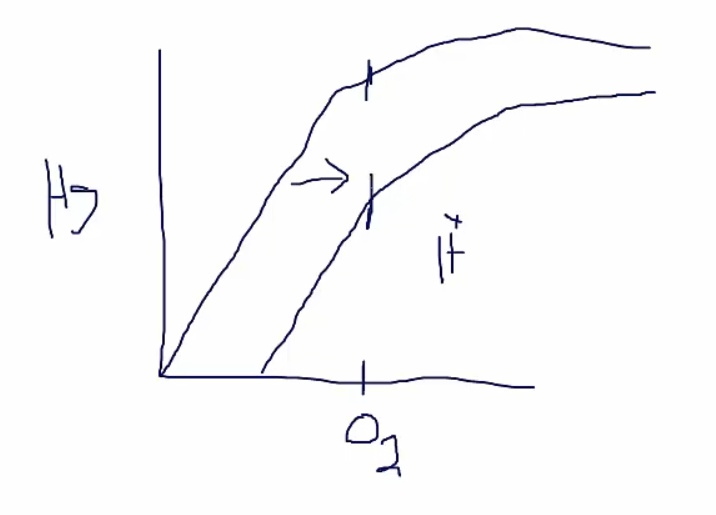
- less saturation at any level
- H push O2 off hemoglobin
- O2 more easily leave
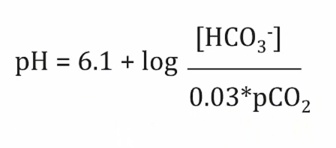
Alkalosis

- shift curve to left
Acid Base Problems
Examples
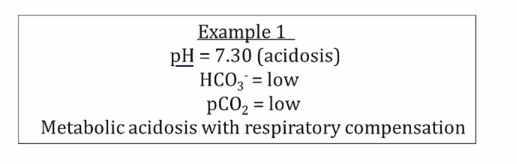
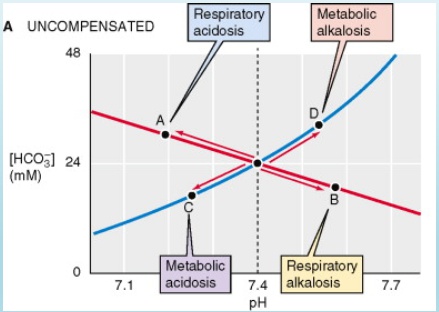
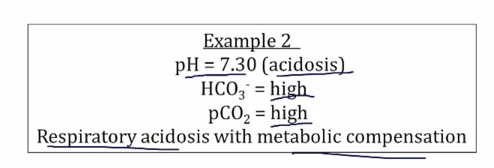

Compensation

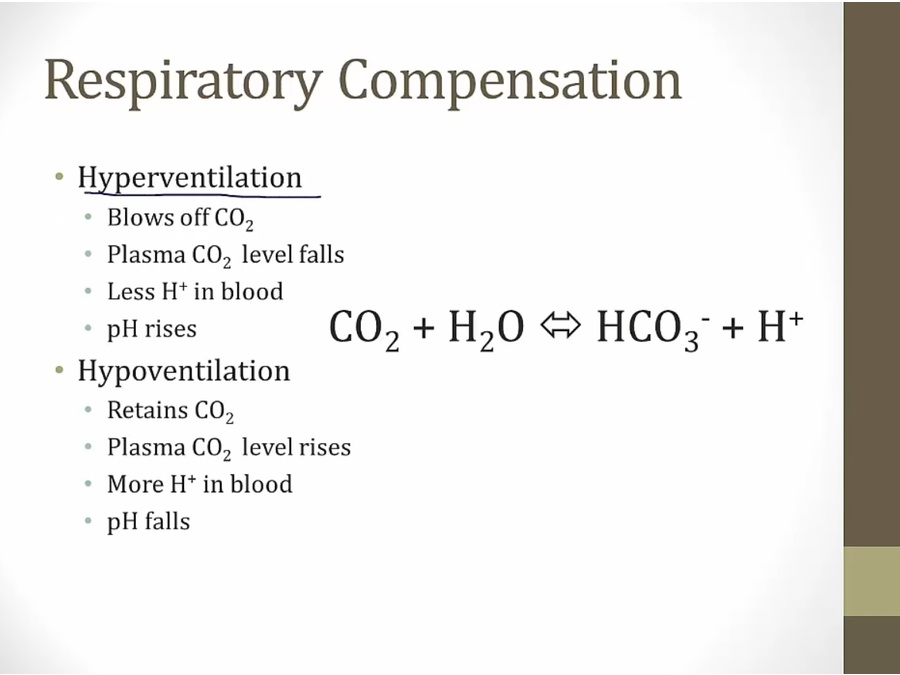

- buffers prevent low pH from killing kidney

- vomiting: alkalotic. Diarrhea: acidotic
Mixed Disorders

Metabolic Acidosis
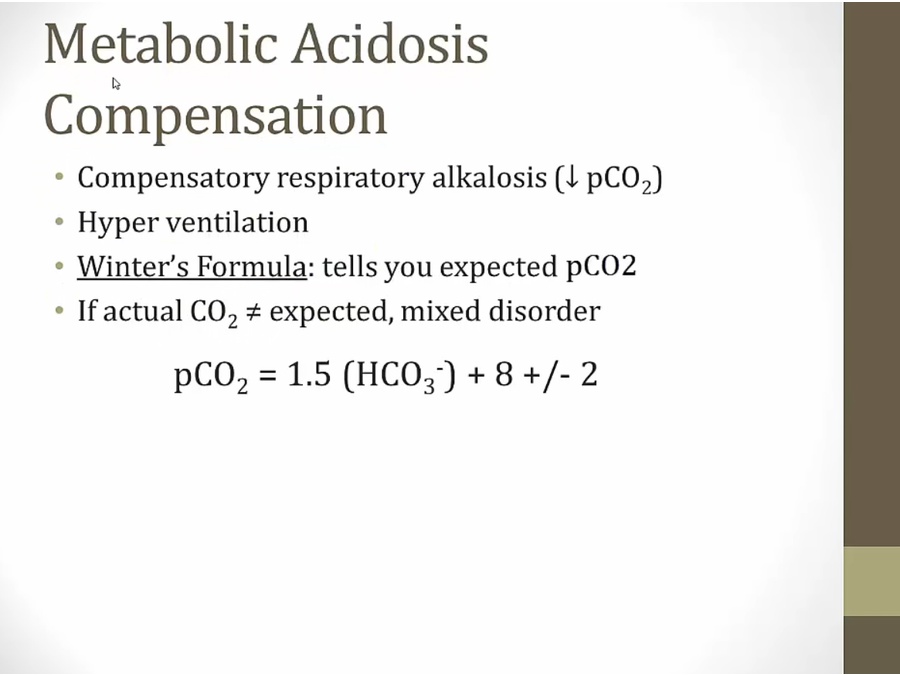
- any kind of meta acidosis, compensation is respiratory alka
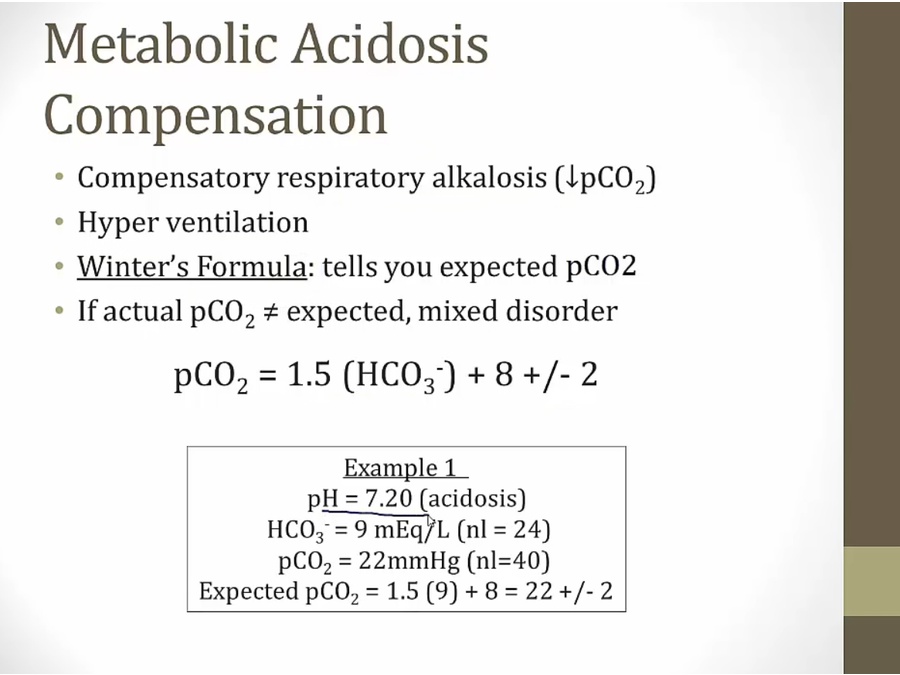
- simple metabolic acidosis with no secondary disorder
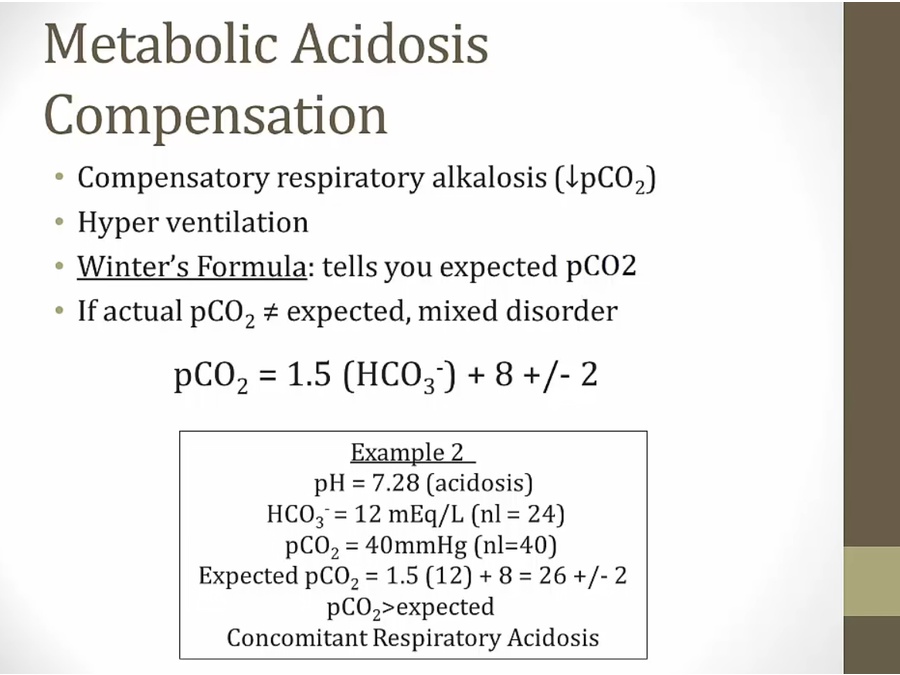
Metabolic Alkalosis
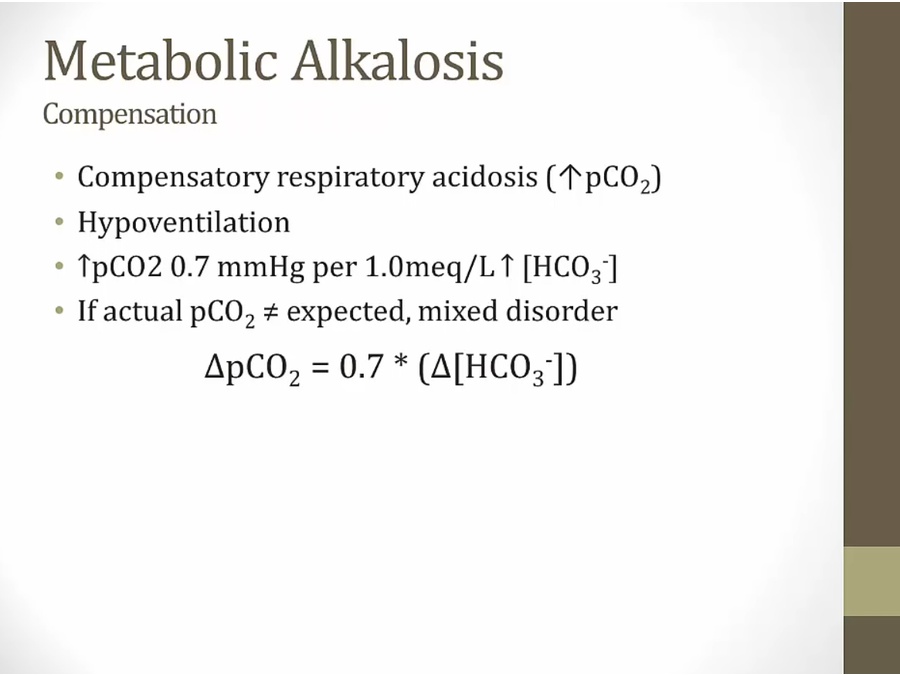
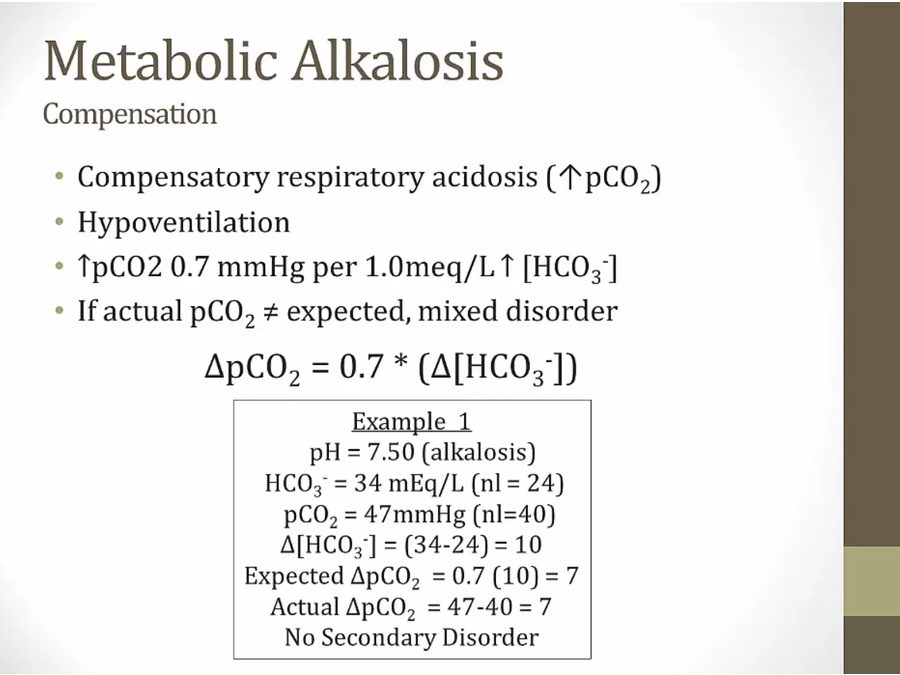
Respiratory acidosis

- acute: within minutes, intracellular buffer soaks up acid via hemoglobin, more HCO3
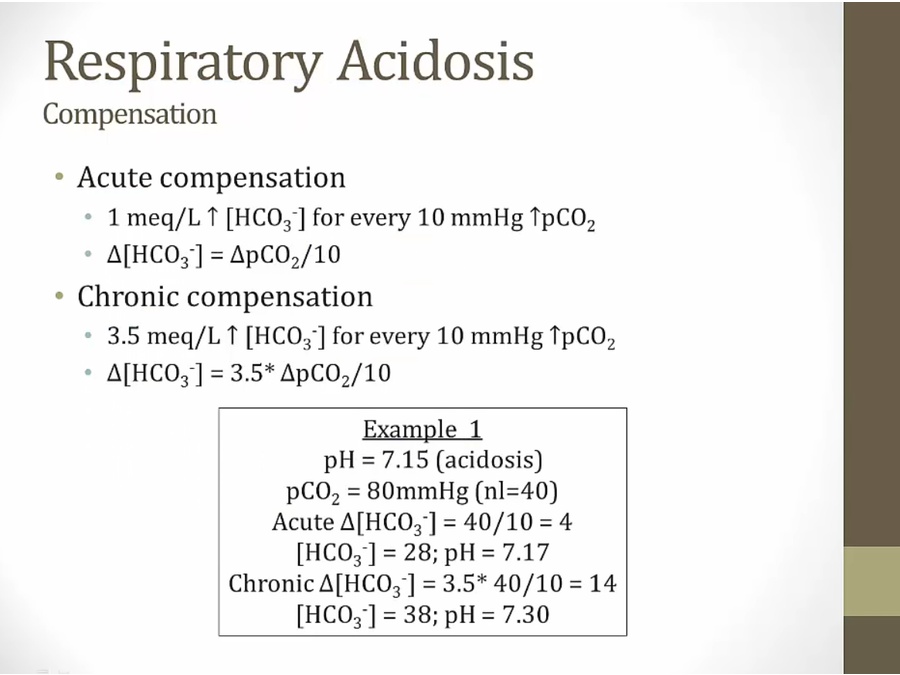
- low yield. Don’t memorize
Respiratory alkalosis
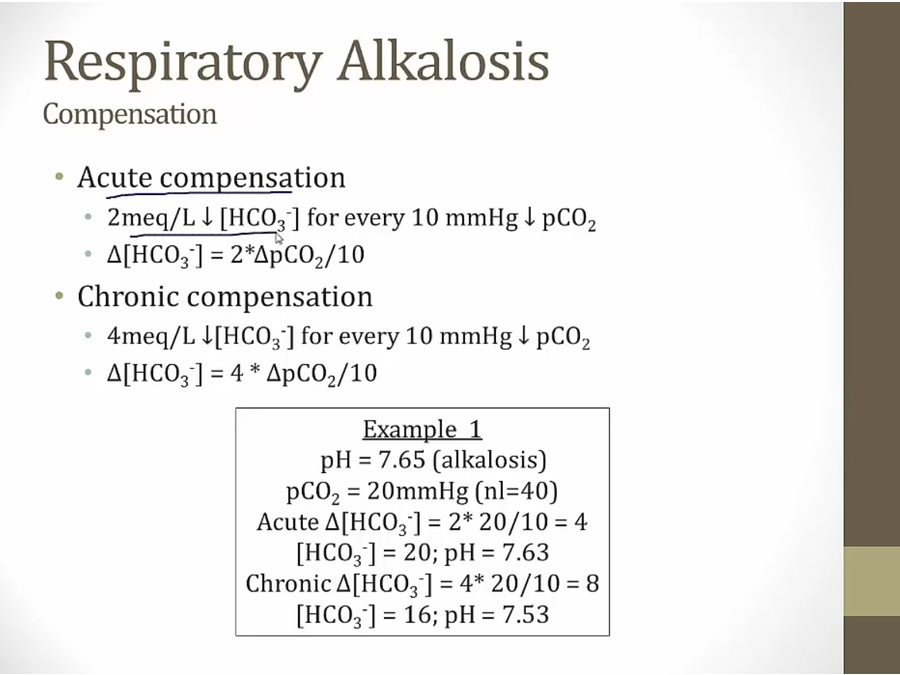
- high altitude, respiratory alkalosis
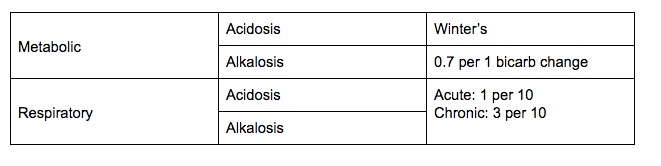

- special: respiratory acidosis and metabolic alkalosis or metabolic acidosis and alkalosis