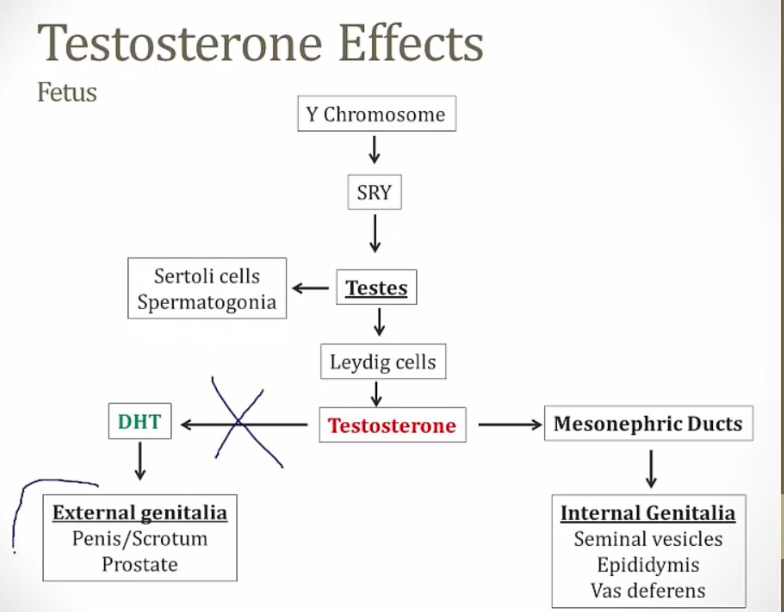
Disorders of Sexual Differentiation
Pseudohermaphroditism
Male or female karyotype but ambiguous genitalia.
Caused by excess/deficient androgen:
- Hypopituitarism
- Congenital adrenal hyperplasia
- Androgen insensitivity
- 5 alpha reductase deficiency
- Aromatase deficiency
Female Pseudohermaphroditism
Is due to excessive and inappropriate exposure to androgenic steroids during early gestation (e.g, congenital adrenal hyperplasia or exogenous administration of androgens during pregnancy).
- Karyotype is 46, XX
- Ovaries are present. No testes
- External genitalia are virilized or ambiguous .
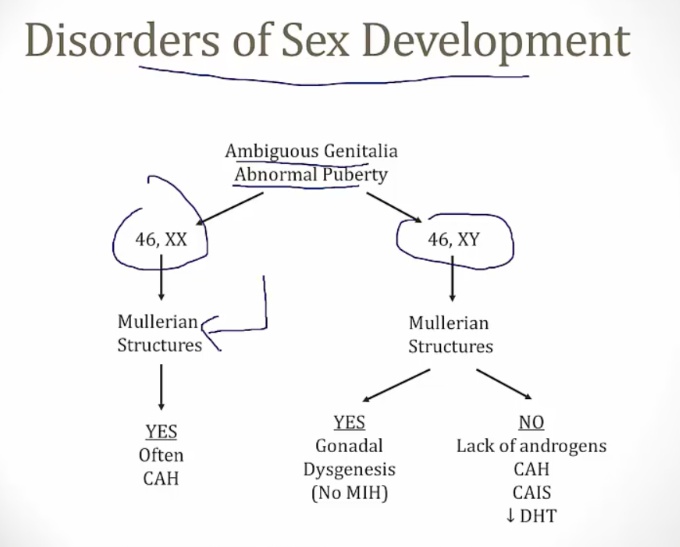
Male Pseudohermaphroditism
Is most commonly caused by androgen insensitivity syndrome (testicular feminization).
- Karyotype is 46, XY
- Testes are present
- External genitalia are female or ambiguous.

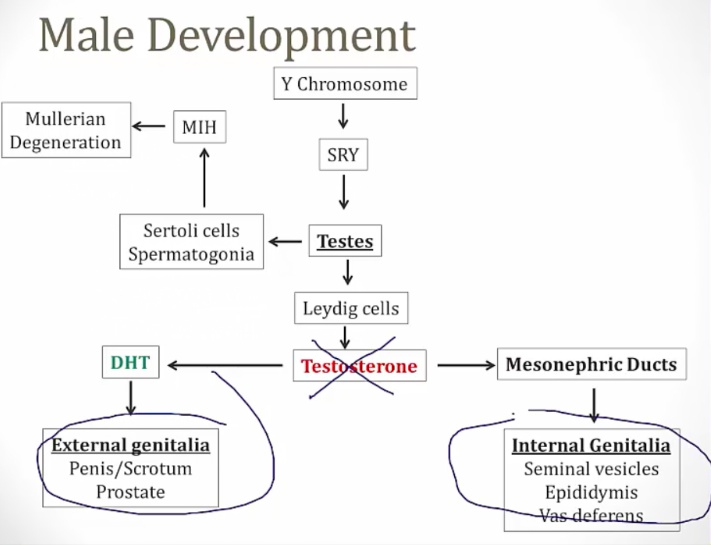
Androgen Insensitivity Syndrome
Formerly testicular feminization.
Is due to impaired androgen receptors.
- SRY gene is present: regression of Mullerian structures
- Testosterone is present, receptor not responsive. No internal genitalia
- No DHT, which is required for external male genitalia development.
- Result: female external genitalia with scant sexual hair and a rudimentary vagina; uterus and fallopian tubes are absent.
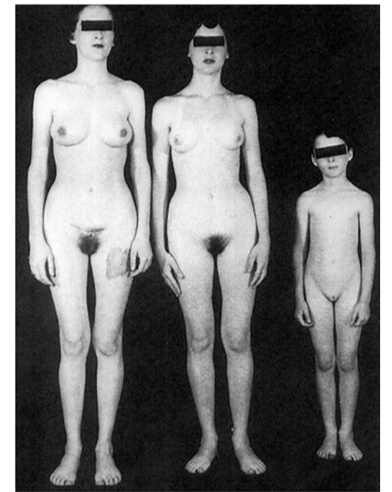
At birth:
- No ovaries; testes formed in utero and undescended (SRY gene) (inguinal mass)
- No uterus/fallopian tube (MIH from sertoli cells)
- female external genitalia (no testosterone)
Puberty:
- Appears female with amenorrhea
- Breasts develop (testosterone to estrogen)
- No armpit/pubic hair (lack testosterone) (differentiate from imperforate hymen that has hair)
- Amenorrhea (no uterus)
- No cervix and rudimentary vagina on exam
- Testes in abdomen or labia majora.
Methods for diagnosis:
- Karyotyping
- Androgen receptor mutation analysis
- Normal FSH levels
- Increased levels of testosterone, LH, and estrogen..
Orchidectomy is recommended in patients to reduce the risk of malignancy.
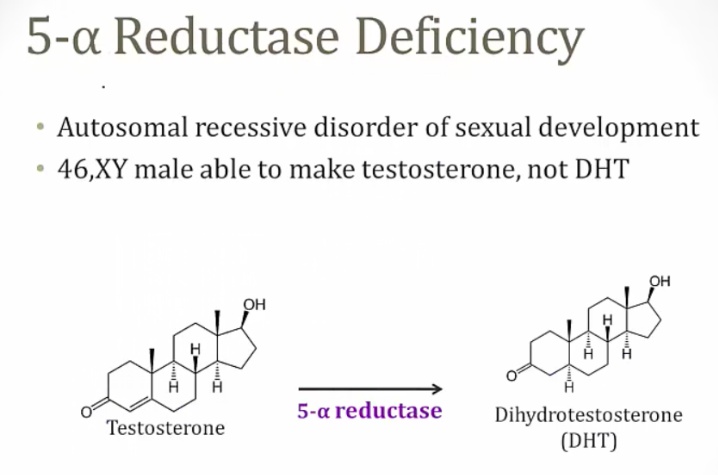
5-a reductase deficiency
An autosomal recessive disorder resulting in an inability to convert testosterone to dihydrotestosterone (DHT).
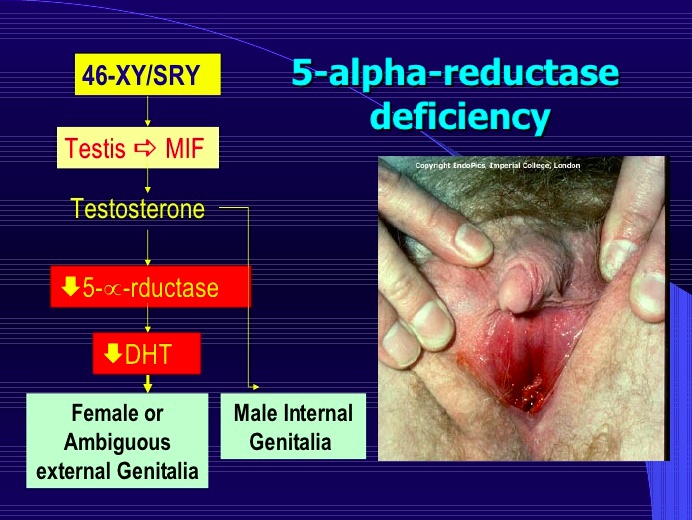
At birth:
- Normal internal genitalia (epididymis, vas, seminal)
- ambiguous genitalia (blind ending vagina) until puberty
- however the external genitalia are female and patients are often raised as females
- can have hypospadias (small penis)
During puberty:
- Female masculinize due to Leydig cells making more testosterone
- Normal testosterone serum level
- Absent uterus, blind vagina, undescended testes
- Masculinization and virilization of the external genitalia (enlargement of the phallus, increases in body hair and muscularity, voice deepening, no breast development) occurs due to increasing levels of testosterone
- grows a penis at puberty.
Internal genitalia are normal: presence of the SRY gene on the Y chromosome encodes Leydig cells which produce normal levels of testosterone that stimulate development of Wolffian structures. They also encode for Sertoli cells, which produce AMH/MIF that leads to normal regression of the Mullerian duct structures.
Lab findings:
- Testosterone and estrogen levels are normal
- LH may be normal or increased.
Diagnosis is done by:
- Karyotyping
- Abdominopelvic ultrasound for presence of female or male internal structures
- Elevated serum ratio of testosterone to DHT (hallmark of diagnosis).
Treatment includes two phases:
- Phase 1: gender assignment and surgical correction for desired sex
- Phase 2: hormone replacement therapy that can be tailored to the chosen sex..
Aromatase Deficiency
The inability to synthesize estrogens from androgens.
Masculinization of female (46, XX) infants produces ambiguous genitalia
- High serum testosterone and androstenedione
- Can present with maternal virilization during pregnancy due to the fetal androgens crossing the placenta.
True Hermaphroditism
Previously known as True Hermaphroditism.
Defined by the presence of both testicular and ovarian tissues in a single individual. This affects the development of external and internal genitalia in a variable manner.
Various genes have been implicate, including:
- RSPO1 gene
- SRY gene
- DMRT gene.
A 46, XX karyotype is most commonly seen. The 47, XXY karyotype can also be seen, but it is less common..
Patients are born with ambiguous genitalia. Most patients will undergo breast development during puberty. Physical exam findings include:
- Uterine abnormalities
- Abnormal vagina
- Chordee penis (if penis is present)
- Urogenital sinus
- Hypospadias
- Bifid scrotal folds.
Diagnosis:
- Chromosomal analysis
- Hormone testing
- Imaging to find occult gonads..
Definitive diagnosis can be made with gonadal biopsy.
Treatment options involves:
- Sex hormone replacement if delayed puberty is seen
- Surgery may be indicated to reinforce the decided upon gender assignment..
Sex Chromosome Disorder
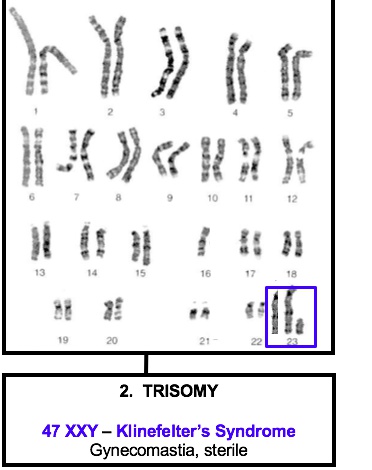
Klinefelter’s syndrome
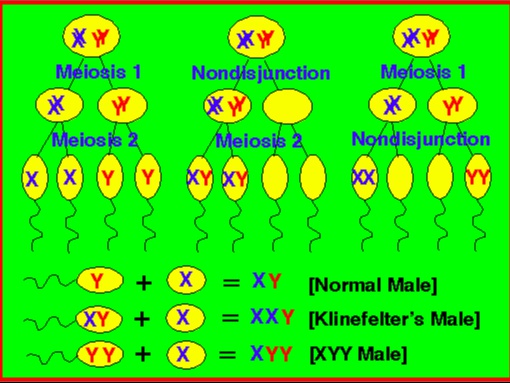
47, XXY males is a sex chromosome disorder caused by meiotic non-disjunction (most commonly in meiosis I).
The manifestations are the result of the following hormonal abnormalities:
- Dysgenesis of the seminiferous tubules: no inhibin and high FSH
- Damaged Leydig cell: no testosterone
- Increase in FSH: increased aromatase synthesis, conversion to estrogen.
The primary reason for hypogonadism and feminization is that testosterone does not have a normal interaction with androgen receptors, which along with increased conversion into estradiol, causes hypogonadism and feminization.
The clinical presentation is variable, but includes:
- Low testosterone: Testicular atrophy, infertility
- High estrogen: Eunuchoid appearance (wide hips and gynecomastia), Female distribution of body hair
- Long extremities
- Low testosterone: Lack of male secondary sex characteristics (deep voice, beard, male pubic hair distribution)
- May present with developmental delay.
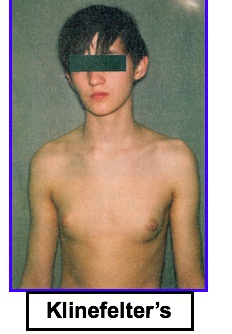
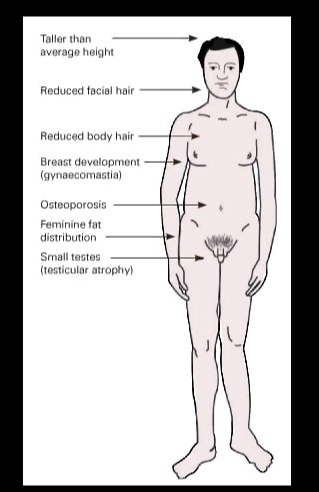
Diagnosis is confirmed with a karyotype showing 47, XXY.
- Buccal smear preps usually show a Barr body (an inactivated X chromosome).
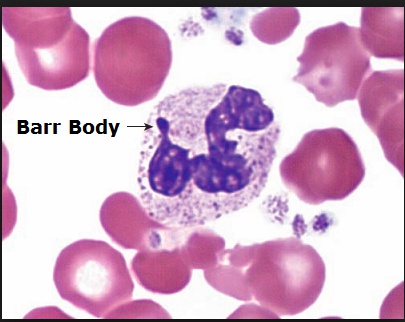
Patients often have complications including:
- Increased incidence of Type II Diabetes Mellitus and metabolic syndrome
- Mitral valve prolapse
- Osteoporosis/Bone Fractures.
Can be managed with the following:
- Testosterone replacement therapy
- Surgical removal of breast tissue
- Psychiatric therapy to assist the patient with coping..
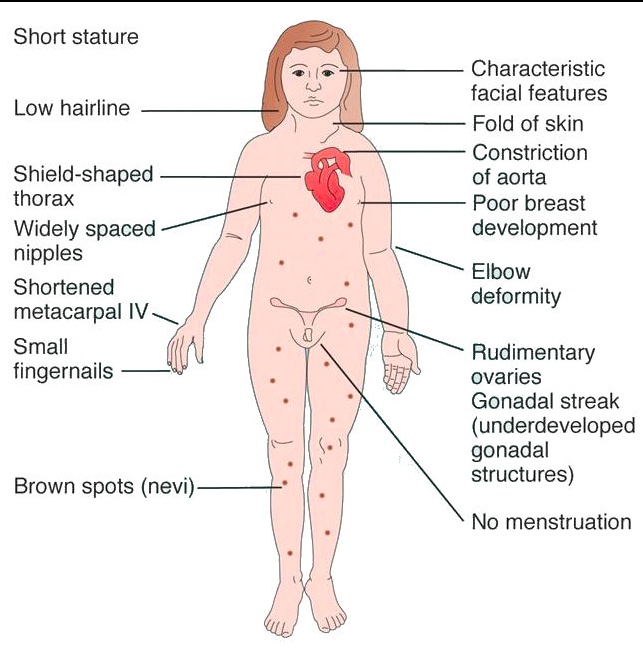
Turner
45, XO females, is the most common genetic cause of primary amenorrhea.
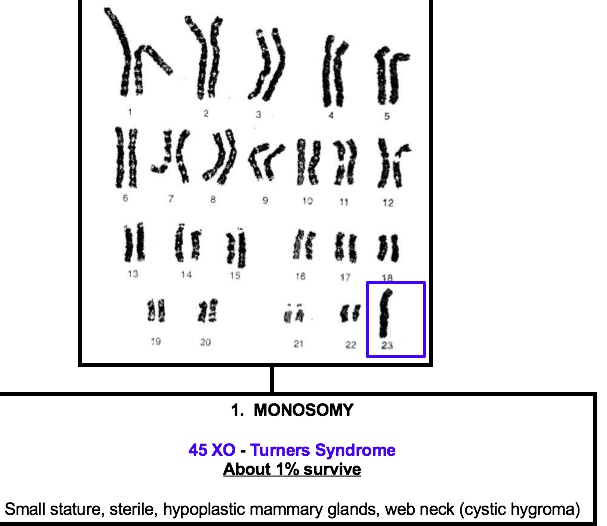
Can be caused by:
- Meiotic non-disjunction (60% of cases): 45,XO
- Mosaicism (30% of cases): 45,XO/46,XX
- Structural abnormalities of the X chromosomes (10% of cases)..

The clinical presentation includes the following:
- Short stature (<5 feet) due to deletion of second SHOX gene on the X chromosome
- Streak gonads (ovaries are replaced with fibrous tissue)
- Infertility
- Menopause before menarche
- Broad chest with widely-spaced nipples
- Webbing of the neck (cystic hygroma) with a low hairline
- Lymphedema in the hands and feet
- Abnormal development of female secondary sex characteristics.
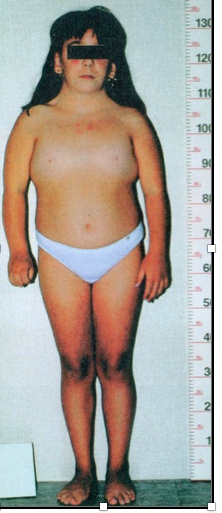
cystic hygroma: lymphatic systems not completely developed
swollen areas, neck
Hormonal testing would reveal:
- Decreased estrogen and progesterone
- Increased FSH and LH..
Complications associated include:
- Preductal coarctation of the aorta
- Bicuspid aortic valve
- Hypothyroidism
- Horseshoe kidney
- Ovarian dysgerminoma..
Pharmacological treatment includes:
- Growth hormone to correct short stature
- Estrogen therapy in order to achieve adult sexual development..
Although rare, pregnancy is possible in some patients with Turner Syndrome. In those cases, they must become pregnant via in vitro fertilization using donor oocytes, exogenous estradiol-17beta and progesterone. It is important to remember that although these patients do not have working ovaries, in most cases they do have a normal uterus with an endometrium that responds normally estrogen; thus making pregnancy possible..
Double Y
47, XYY is found in 1:1000 males and is often undetected.
Is caused by a random event of paternal nondisjunction during anaphase II of meiosis II..

patients will be phenotypically normal, but will present with tall stature and severe acne.
- Normal testosterone levels, and most have normal sexual development and normal fertility.
- Typically have normal behavior and intelligence. However, in some cases they have been associated with aggressive behavior, learning disability, and autism spectrum disorders.
Fragile X
The second most common genetic cause of intellectual disability (Down syndrome is the most common).
Is caused by expanded trinucleotide CGG repeats in the promoter segment of the FMR1 gene. The expanded promoter segment enhances methylation leading to silencing of the FMR1 gene.
- Silencing of the FMR1 gene leads to decreased synthesis of FMRP protein
- Lack of FMRP is what causes the clinical symptoms seen in Fragile X syndrome
Is more common in males and presents with:
- Macro-orchidism (enlarged testes)
- Large jaw
- Large everted ears
- Mental retardation
- The most common cardiac complication is mitral valve prolapse.
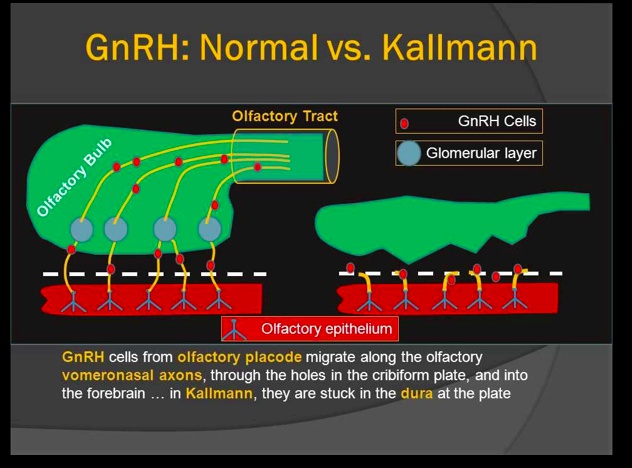
Kallmann syndrome
A form of hypogonadotropic hypogonadism, which results in a failure to complete puberty.
It is associated with anosmia.
Is also known as hypogonadotropic hypogonadism with anosmia.
Is caused by failed migration of GnRH neurons from the olfactory placode to the hypothalamus, which results in improper development of the olfactory bulb (explains the anosmia).
Without GnRH neurons, the hypothalamus synthesizes and secretes decreased amounts of GnRH leading to decreased LH, FSH, and testosterone/estrogen (which accounts for hypogonadism).
Presents with the following symptoms:
- Anosmia
- Lack of secondary sex characteristics
- Lack of testicular development in males
- Infertility (low sperm count in males; primary amenorrhea in females)
- Failure to start/complete puberty.
Is treated with sex hormone replacement therapy in order to achieve normal adult sexual development in patients. Puberty can be induced with the administration of LH and FSH..