Hepatitis B
Acute Infection
- sx:
- 70% asymptomatic
- 30% nausea, anorexia, jaundice, RUQ pain. Resolve spontaneously
- 0.5% ALF
- labs:
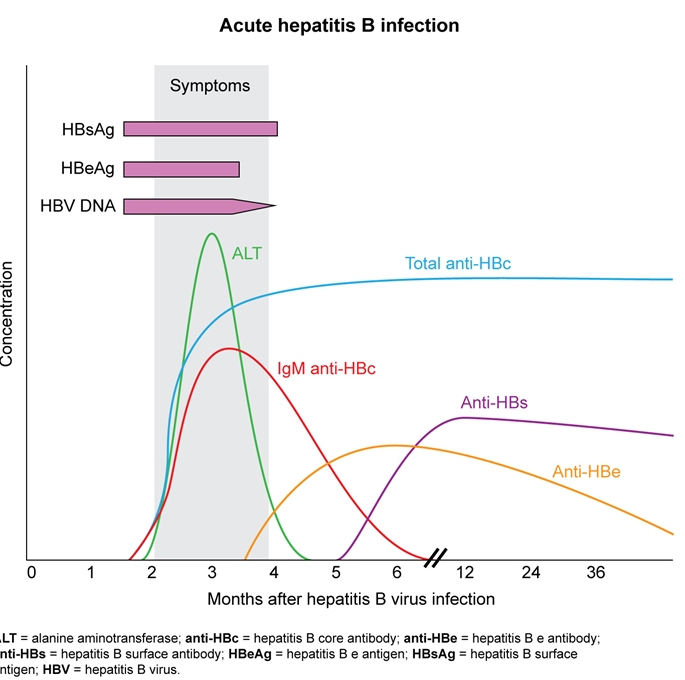
- ALT elevation (as high as 3000 u/L)
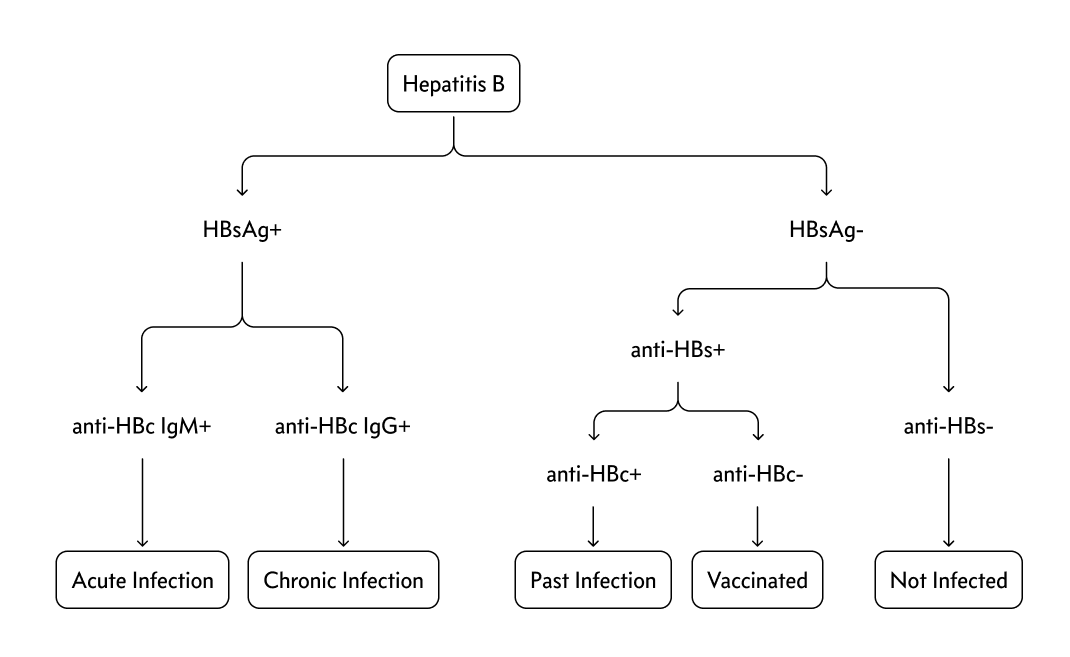
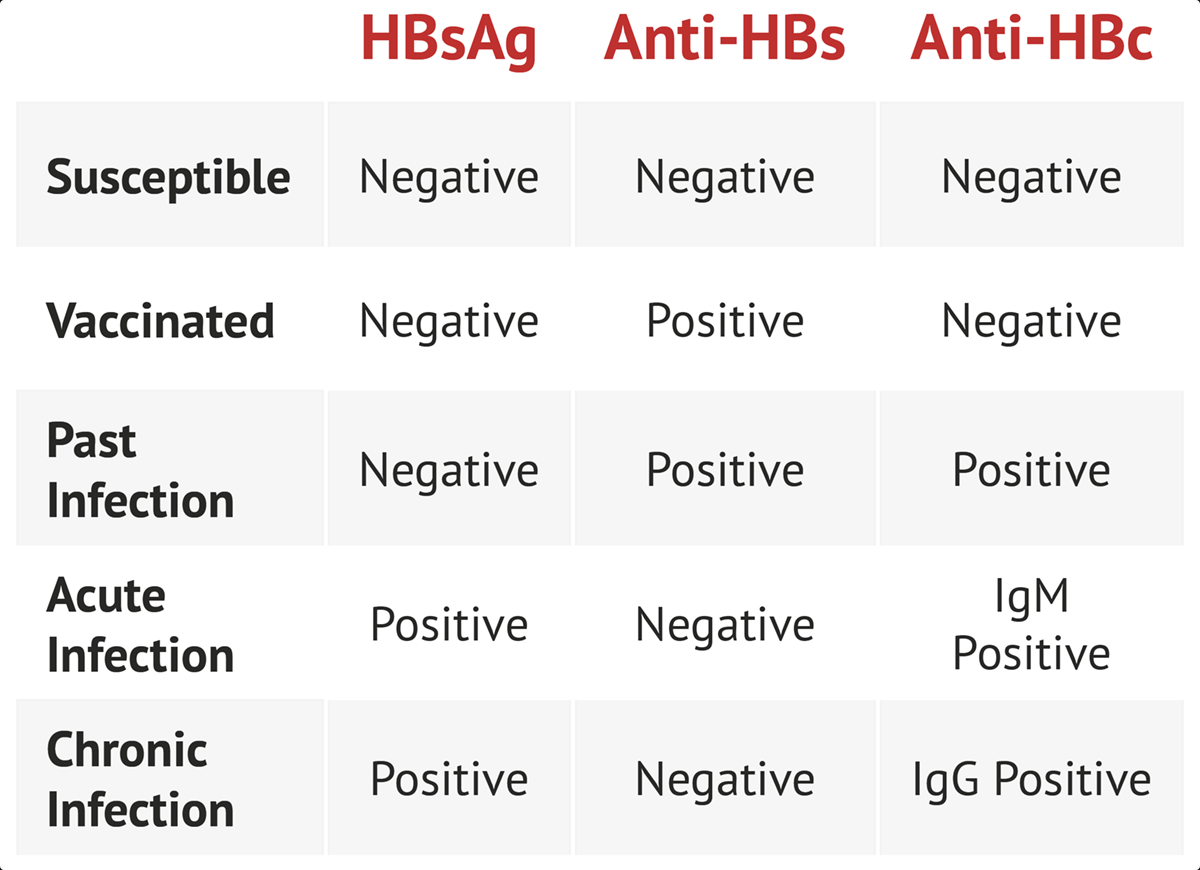
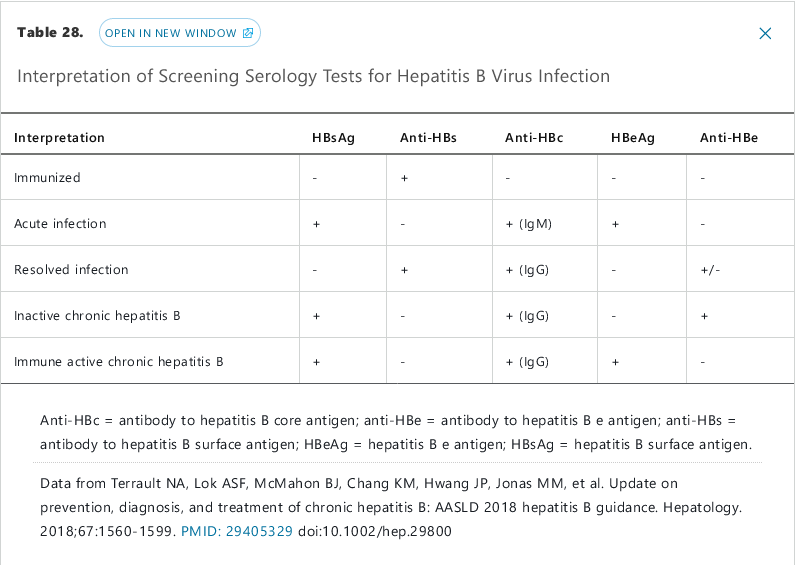
- +hep B surface ag
- +hep B IgM antibody
- +hep B e ag (high infectivity)
- detectable B DNA
- treatment:
- self limited. Milder onset resolve in days. More severe sx may persist for 1-4 months
- outpatient observation
- hospitalization: severe illness, unstable, encephalopathy
- antiviral not shown to change course of infection
- antiviral recommended for patients with immunosuppression, concurrent hep C or D, hepatic failure
- chronic HBV: patients who do not clear hep B surface ag after 6 months
For patients with acute HBV infection, antiviral treatment (eg, tenofovir, entecavir) should be considered only in those with a severe (eg, INR >1.5) or prolonged (eg, >4 weeks of symptoms with significant jaundice and bilirubin >3 mg/dL) course or with acute liver failure (to reduce posttransplant reinfection risk). Although this patient with acute HBV infection has bilirubin >3 mg/dL, he has not had >4 weeks of symptoms and has no other indications (eg, liver failure, hepatic encephalopathy) for treament (Choice D).
Chronic HBV develops following acute infection in up to 5% of adults; it is defined by persistent HBsAg for >6 months and can be active or inactive. Chronic inactive HBV is characterized by a negative HBeAg, normal alanine aminotransferase (ALT), and <2,000 IU/L HBV DNA and does not require treatment. Patients with active chronic HBV (elevated HBV DNA, ALT >2x normal, and HBeAg positive) can be considered for antiviral treatment, as can the subset of patients with occult chronic HBV infection (negative HBeAg but elevated HBV DNA). This patient with 1 week of symptoms has acute, not chronic, HBV infection.
Transmission
- vertically
- newborns have highest risks (90%)
- sexually
- percutaneously
- close person to person contact
Testing
- high rates: Asia, Africa, the South Pacific, European Mediterranean countries, Eastern Europe, most of South America, Honduras, Guatemala, and the Middle East (except Israel and Cyprus)
- U.S.-born persons not vaccinated as infants whose parents were born in endemic areas
- Household or sexual contact with hepatitis B surface antigen–positive persons
- Intravenous drug use
- Multiple sex partners or history of sexually transmitted infection
- Men who have sex with men
- History of incarceration
- History of hepatitis C virus or HIV infection
- Hemodialysis
- Pregnancy
- Elevated aminotransferase levels of unknown cause
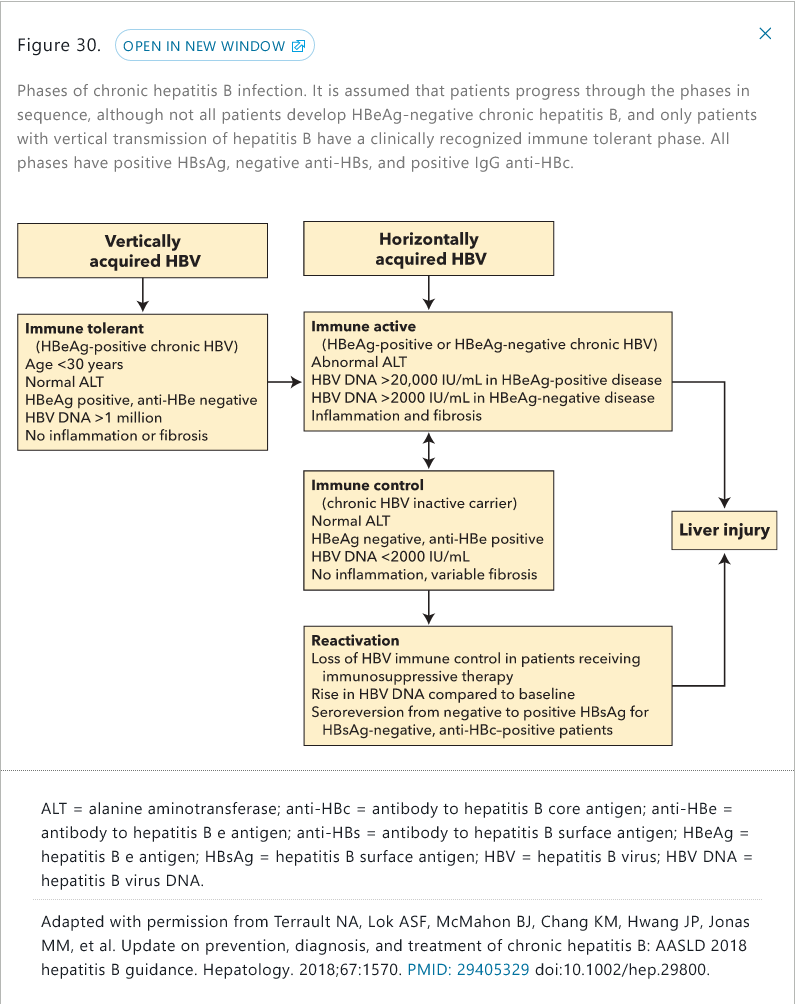
Phases
- immune tolerant
- vertically transmission. 2-3 years. No treatment except > 40 and DNA > 1 million IU/mL
- eventually transitions to immune-active
- immune active
- high ALT, intermittent or persistent
- HBV DNA > 20,000 IU/mL in HBeAg+ and > 2000 in HBeAg-
- treatment warranted
- immune control (inactive chronic HBV infection)
- spontaneous seroconversion occurs at 10%/year
- ALT must be normal
- loss of HBeAg and development of antibody
- reactivation
- loss of immune control
- usually from immunosuppression
- seroconversion from HBsAgto +
Treatment is advised for patients with acute liver failure, infection in the immune-active phase or reactivation phase, and cirrhosis, and in selected immunosuppressed patients. First-line treatment is entecavir or tenofovir. Lamivudine, adefovir, and telbivudine are less commonly used due to resistance. Pegylated interferon can be used for 48 weeks in patients with high ALT levels, low HBV DNA levels, and without cirrhosis. Candidates for interferon are those who have a desire for finite therapy, are not pregnant, and do not have significant psychiatric disease, cardiac disease, seizure disorder, cytopenia, or autoimmune disease.
Treatment goals for patients in the HBeAg-positive, immune-active phase are HBeAg loss and anti-HBe seroconversion, which should be followed by an additional 12 months of treatment. Goals of treatment in the HBeAg-negative, reactivation phase are HBV DNA suppression and ALT normalization; oral antiviral agents are generally continued indefinitely. Patients with cirrhosis should continue oral antiviral medications indefinitely. HBsAg seroconversion rarely occurs with oral antiviral treatment and, therefore, is not a goal of treatment. Regression of fibrosis and even of cirrhosis can occur with treatment.
Rarely, patients with HBV infection develop membranous nephropathy, membranoproliferative nephropathy, polyarteritis nodosa or cryoglobulinemia, which should prompt treatment with oral antiviral therapy.
The survival rate after liver transplantation for end-stage liver disease from HBV infection is greater than 90% at 1 year. Recurrence of HBV infection in transplant recipients is prevented with HBV immunoglobulin and/or oral antiviral therapy.
The prognosis for untreated individuals with HBV infection worsens with age, particularly with age older than 40 years. Approximately 40% of deaths in HBV-infected persons older than age 40 years are related to hepatocellular carcinoma or decompensated cirrhosis. The following characteristics are associated with an increased risk for hepatocellular carcinoma in patients with HBV infection and are indications for surveillance with ultrasound or cross-sectional imaging every 6 months: (1) cirrhosis; (2) Asian descent plus male sex plus age older than 40 years; (3) Asian descent plus female sex plus age older than 50 years; (4) sub-Saharan African descent plus age older than 20 years; (5) persistent inflammatory activity (defined as an elevated ALT level and HBV DNA levels greater than 10,000 IU/mL for at least a few years); and (6) a family history of hepatocellular carcinoma.