Uworld Goodpastures
- related: Uworld Questionnaires
- tags: #literature
Anti–glomerular basement membrane (anti-GBM) antibody disease is a rare form of RPGN with an incidence of less than one case per million per year. Anti-GBM antibody disease accounts for about 20% of RPGN cases in adults. The lesion is more frequently seen in White patients and has a bimodal age and gender distribution, with peak incidences in young men in the second and third decades of life and in older women in the sixth and seventh decades of life. More than half of patients with anti-GBM antibody disease present with RPGN and pulmonary hemorrhage. About one third of patients present with isolated glomerulonephritis. Notably, up to one third of patients have concurrent circulating ANCA (usually antimyeloperoxidase, MPO) antibodies.
Circulating anti-GBM antibodies target the alpha-3 chain of type IV collagen. When anti-GBM antibodies bind rapidly and tightly to the GBM, they incite an intense inflammatory response that translates to the typically fulminant nature of this disease.
Clinical Manifestations
The presentation of anti-GBM antibody disease is similar to that of other forms of RPGN previously described, with macroscopic or microscopic hematuria, erythrocyte casts, varying ranges of proteinuria, and usually moderate to severe AKI. Lung involvement (Goodpasture syndrome) occurs in >50% of patients; hemoptysis can be a presenting symptom, although shortness of breath or cough should also raise suspicion for a pulmonary-renal syndrome even in the absence of hemoptysis.
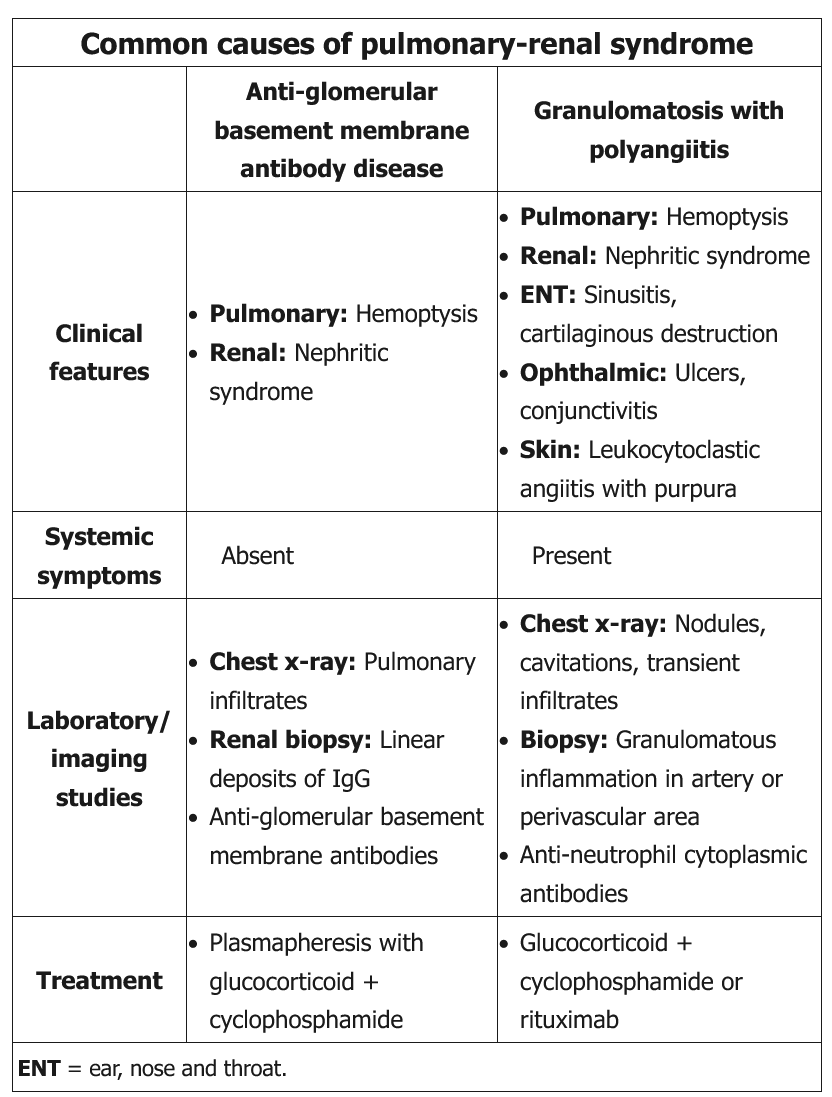
This patient has evidence of pulmonary-renal syndrome (pulmonary hemorrhage and glomerulonephritis), which is commonly caused by anti–glomerular basement membrane (GBM) antibody disease (Goodpasture syndrome) or by granulomatosis with polyangiitis. His lack of constitutional vasculitic symptoms (eg, fever, weight loss, arthralgia, fatigue) makes anti-GBM antibody disease more likely.
Patients with anti-GBM antibody disease typically have a progressive glomerulonephritis (eg, hematuria, red blood cell casts, proteinuria, hypertension) and acute renal failure. Pulmonary involvement occurs in up to 60% of patients and presents with dyspnea, cough, and hemoptysis with pulmonary infiltrates on chest x-ray.
Diagnosis is suggested by positive serum anti-GBM antibody and is confirmed by renal biopsy showing linear immunofluorescence of IgG in glomerular capillaries. Plasmapheresis is the preferred treatment to remove circulating antibodies and is combined with corticosteroids and cyclophosphamide to prevent formation of new antibodies.
Compared to anti-GBM antibody disease, granulomatosis with polyangiitis is an inflammatory vasculitis and therefore more likely to cause constitutional vasculitic symptoms; in addition, ear, nose, and throat and ocular manifestations are common (Choice D).
Diagnosis
Kidney biopsy in anti-GBM disease shows a crescentic glomerulonephritis on light microscopy and pathognomonic linear staining for IgG along the glomerular capillaries, indicative of antibodies directed against the GBM. Serologic testing for anti-GBM antibodies is performed at the time of diagnosis. The serologic test is done by indirect immunofluorescence or direct enzyme-linked immunoassay (ELISA), with sensitivity ranging from 60% to 100%; therefore, a kidney biopsy to confirm diagnosis is recommended unless contraindicated. Even with a renal biopsy diagnosis, antibody levels should be measured because their titers can be followed to assess efficacy of therapy.
Treatment and Prognosis
Outcomes in anti-GBM disease are based largely on the degree of AKI at the time of diagnosis and treatment. In an oft-cited series, patients with anti-GBM disease who presented with serum creatinine <5.65 mg/dL (500 µmol/L) had, at 10 years, a <5% overall mortality and a <20% risk for ESKD. In contrast, patients who presented with dialysis-dependent kidney failure had a 35% mortality rate within the first year of diagnosis, and only 8% were able to be taken off dialysis during this time. Treatment for anti-GBM disease consists of plasmapheresis, pulse glucocorticoids (high doses of intravenous glucocorticoids over a short period of time) followed by oral prednisone, and cyclophosphamide.