attributable risk
- related: Biostats and Study Design
- tags: #literature #pulmonology
In a small observational study, 100 industrial workers are followed for one year to assess for the development of respiratory symptoms (defined as productive cough lasting at least one week). 30 of 60 smokers experience respiratory symptoms over the year versus 10 of 40 non-smokers. Which of the following is the best estimate of the attributable risk of respiratory disease in smokers?
In a small observational study, 100 industrial workers are followed for one year to assess for the development of respiratory symptoms (defined as productive cough lasting at least one week). 30 of 60 smokers experience respiratory symptoms over the year versus 10 of 40 non-smokers. What percentage of respiratory disease experienced by smokers is attributed to smoking?
In a small observational study, 100 industrial workers are followed for one year to assess for the development of respiratory symptoms (defined as productive cough lasting at least one week). 30 of 60 smokers experience respiratory symptoms over the year versus 10 of 40 non-smokers. What percentage of respiratory disease experienced by all study subjects is attributed to smoking?
Several important topics related to measures of association and impact are covered in this section.
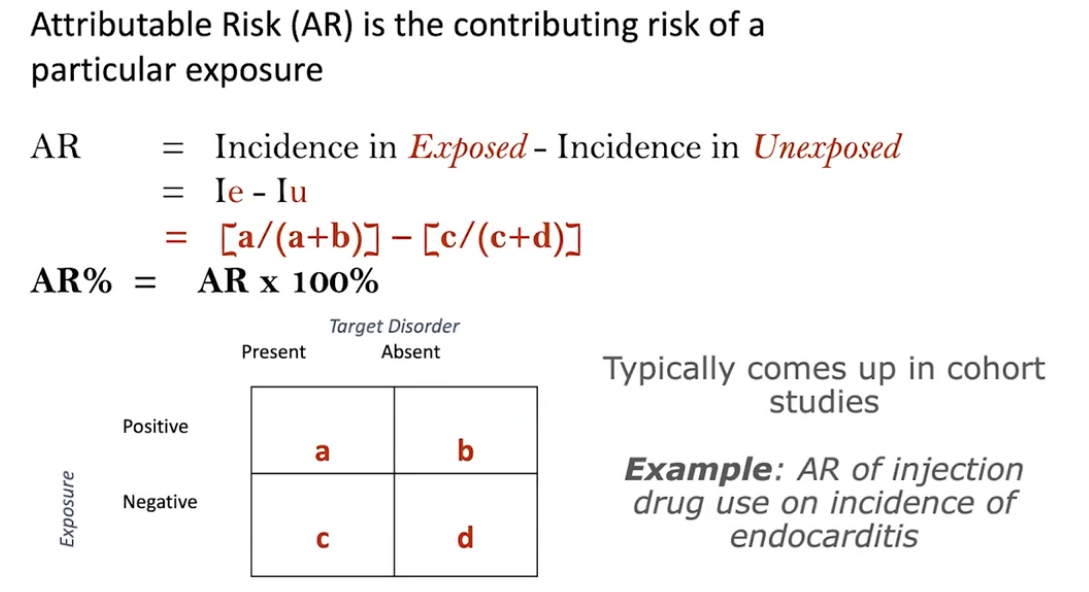
The first topic is known as ‘attributable risk’ or ‘risk difference’. It is a measure of the excess incidence of a disease due to a particular factor (exposure). In Question #1, the one-year incidence of respiratory disease in smokers is 30/60 = 0.5 whereas in non-smokers it is 10/40 = 0.25. The difference between these incidences (0.5 – 0.25 = 0.25) describes the attributable risk. Based on the calculation, we can assume that 25 out of 100 cases of respiratory disease are attributable to smoking.
A related measure known as ‘attributable risk percent’ describes the contribution of a given exposure to the incidence of a disease in relative terms. Attributable risk percent is calculated by dividing the attributable risk by the incidence of the disease in the exposed population (i.e. smokers). In Question #2 we calculate attributable risk percent as follows: (30/60 – 10/40)/(30/60) = 0.25/0.5 = 0.5 (50%). Based on the calculation, we can conclude that 50% of the yearly respiratory disease in smokers is attributable to smoking.
Another measure called population attributable risk percent describes the impact of exposure on the entire study population (in our case, both smokers and non-smokers). To determine population attributable risk percent, first calculate the incidence of the disease in the study population as a whole. In the above study population, there are 30 smokers and 10 non-smokers who develop respiratory disease out of a total of 100 workers (60 smokers and 40 non-smokers). Therefore, the overall incidence of developing respiratory disease in the study population is 40/100. Next, calculate the difference in risk of developing respiratory disease among the study population as a whole and among non-smokers (40/100 – 10/40 = 0.4 – 0.25 = 0.15) and divide this value by the incidence of respiratory disease in the population as a whole (0.15/0.4 = 0.375). Based on the calculation, we conclude that 37.5% of the yearly respiratory disease in the study population is attributable to smoking.
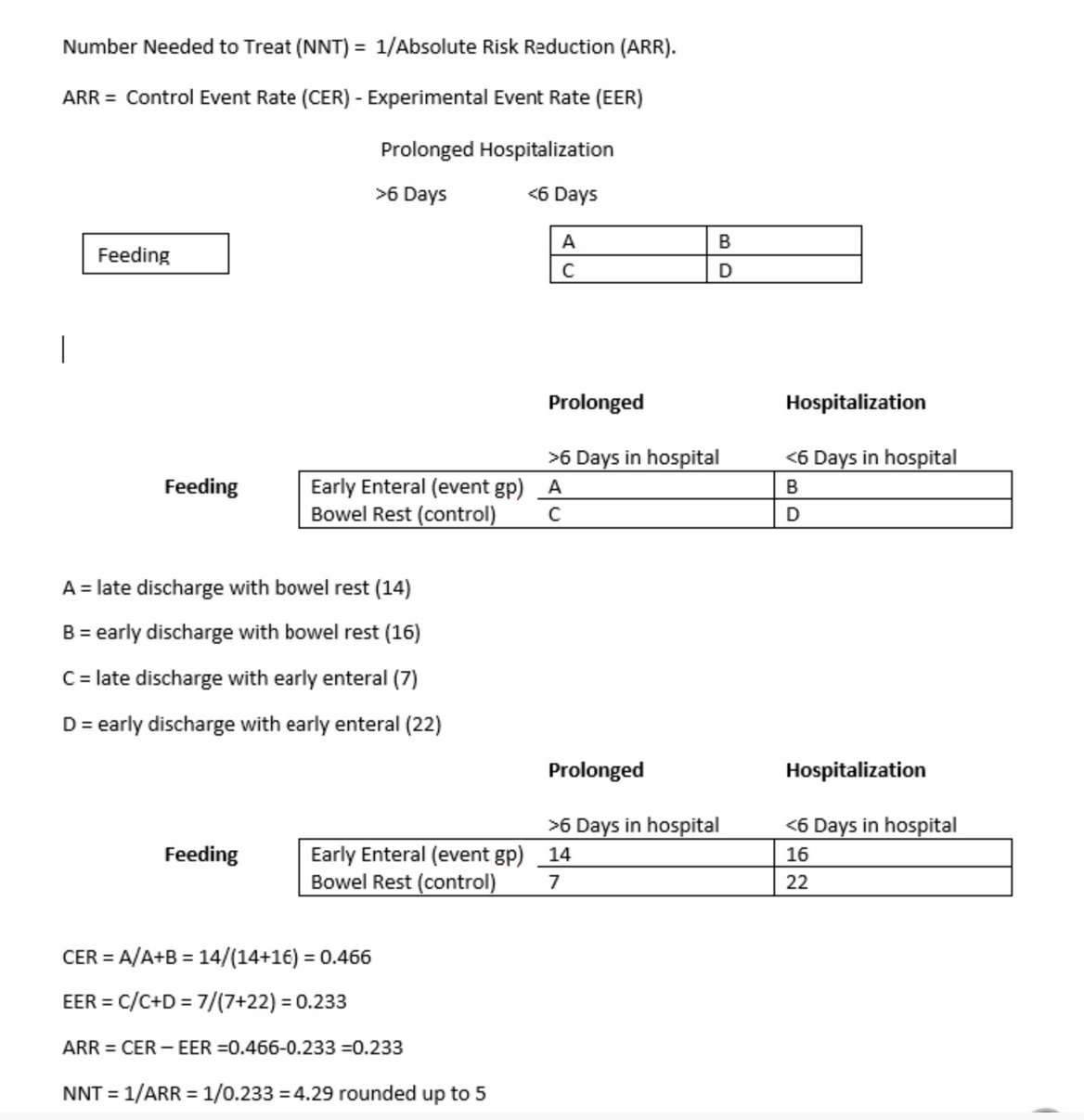
In clinical trials, an important concept related to absolute risk reduction is ‘number needed to treat’ (NNT). It is actually the reciprocal of absolute risk reduction. It answers the following question: how many patients should I treat with the drug (or regimen) of interest to save/extend one life? In Question #4 the death rate in patients placed on the new treatment regimen is 25/50 = 0.5 over 5 years, whereas in patients kept on the conventional chemotherapy regimen the mortality rate is 75/100 = 0.75. The absolute risk difference between the two groups is 0.75 – 0.5 = 0.25. The reciprocal of the absolute risk difference (1/0.25 = 4) reveals the NNT. Based on this result, we can conclude that we need to treat 4 patients with the new regimen as opposed to the conventional regimen in order for one more patient to survive 5 years without relapse.