CAPE COD trial shows steroid reduces mortality in severe pneumonia patients
- related: community acquired pneumonia
- tags: #literature #pulmonology #icu
In patients who are admitted to ICU and have evidence of severe pneumonia and are not treated with vasopressors, use of stress dose steroid for 4 days has 5.6% reduction of absolute risk of mortality. The benefit of steroid is more for patients with CRP > 15 mg/dL (150 mg/L). 1
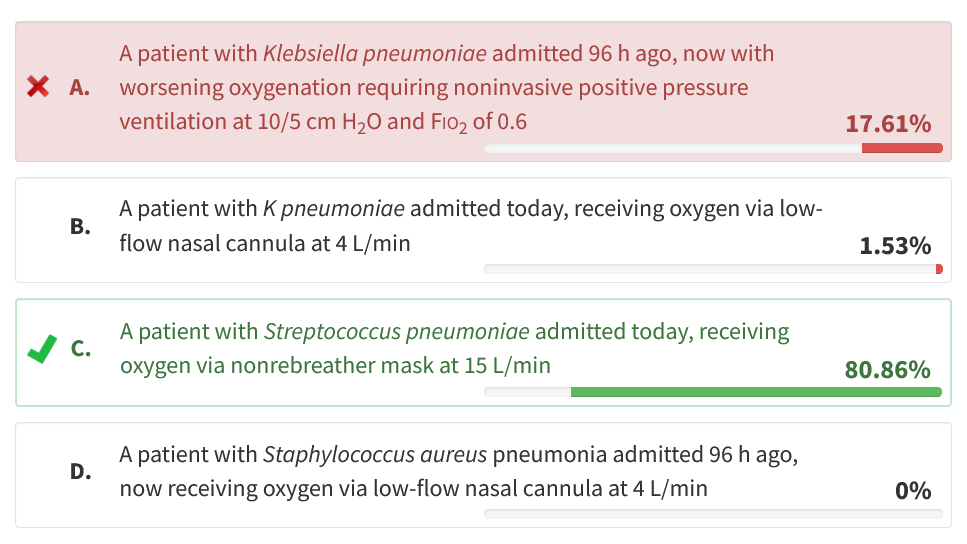
The CAPE COD trial randomly assigned 800 patients with severe CAP in the ICU to receive 200 mg of hydrocortisone per day for 4 to 7 days and then an 8to 14-day taper or placebo. The patients were followed up from 2015 to 2020, but the study was interrupted owing to the COVID-19 pandemic. Severe pneumonia was defined by at least one of the four following criteria: (1) mechanical ventilation with a PEEP of 5 cm H2O or higher, (2) high-flow nasal cannula (HFNC) oxygen with a PaO2/FIO2 less than 300 with FIO2 of at least 0.5, (3) PaO2/FIO2 less than 300 with a nonrebreather (NRB) mask, and (4) a Pneumonia Severity Index score greater than 130. Exclusion criteria were viral pneumonia, septic shock, and a do not resuscitate order. The primary end point of 28-day mortality was reduced in the hydrocortisone group (6.2% vs 11.9%; P = ), and the secondary outcomes of initiation of pressors or need for mechanical ventilation were also reduced. A secondary analysis demonstrated that the difference in 28-day mortality persisted for up to 90 days for patients who received hydrocortisone. Although it is agreed that further studies on the use of adjunctive steroids in severe CAP need to be performed, the recommendation is to start hydrocortisone within 24 h of presentation in patients with severe CAP with acute hypoxemic respiratory failure requiring FIO2 of more than 0.5 via NRB or HFNC. Of the available choices, only the patient in choice C would meet these criteria (choice C is correct).
Another large study by the ESCAPe Study Group randomly assigned patients to methylprednisolone 40 mg/day for 7 days, then tapered over 20 days, but they did not see a morbidity or mortality benefit in the steroid group vs placebo. There were two differences between the studies with respect to time to initiation of steroids. In the CAPE COD study, the participants received steroids within 24 h of admission, and in the ESCAPe study, it took up to 96 h for steroids to be administered. Based on these studies, the recommendation is to administer steroids within 24 h for patients admitted with severe CAP (choice A is incorrect). Although admitted within 24 h, patients B and D do not have severe CAP as described in the CAPE COD study. They are receiving FIO2 of less than 0.5 via nasal cannula and do not require an NRB or HFNC (choices B and D are incorrect).234567
On the basis of the available evidence, which of the following patients with community-acquired pneumonia (CAP) and acute hypoxemic respiratory failure is most likely to benefit from treatment with hydrocortisone?
Links to this note
Footnotes
-
Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31(2):347-382. PubMed ↩
-
Chaudhuri D, Nei AM, Rochwerg B, et al. 2024 focused update: guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Crit Care Med. 2024. PubMed ↩
-
Dequin PF, Meziani F, Quenot JP, et al; CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. PubMed ↩
-
Hydrocortisone for severe community acquired pneumonia in ICU. Drug Ther Bull. 2023;62(1):3. PubMed ↩
-
Meduri GU, Shih MC, Bridges L, et al; ESCAPe Study Group. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022;48(8):1009-1023. PubMed ↩