do not use zanamivir for treatment of COPD due to influenza
- related: Infectious Disease ID
- tags: #literature #pulmonology #icu
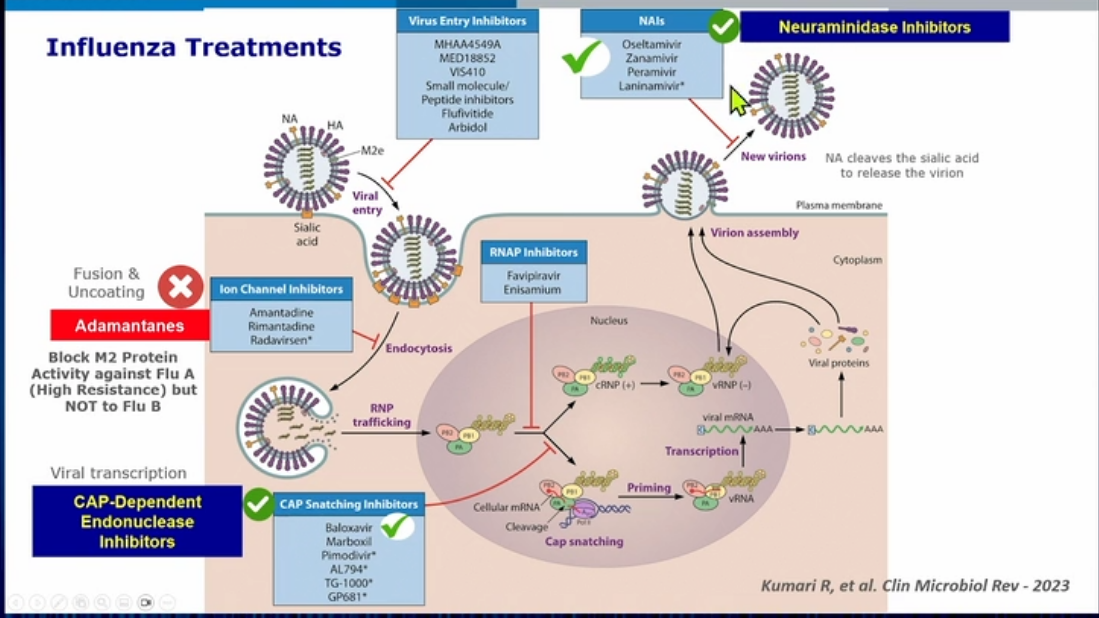
- zanamivir is inhaled and can cause bronchospasm and thus is not recommended for treatment of COPD exacerbation due to flu
- oseltamivir is first line. Can be used for pregnant patients in any trimester or breastfeeding.
- peramivir is IV medication, could be used for ICU sick patients who can’t tolerate enteral oseltamivir. No evidence in pregnant patient.
- amantadine no longer recommended
- baloxavir is for mild infections
Baloxavir marboxil is a Food and Drug Administration (FDA)-approved anti-influenza medication that provides a greater reduction in viral load by 1 day after treatment initiation when compared with oseltamivir (choice D is correct).
Baloxavir marboxil is a prodrug that is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication (choice A is incorrect). This novel mechanism of action differs from that of oseltamivir, which inhibits the neuraminidase enzyme.
Two randomized controlled trials tested the efficacy of baloxavir marboxil as a single dose against placebo and oseltamivir in patients with uncomplicated influenza infection. Baloxavir marboxil reduced the median time to alleviation of symptoms by 26.5 h compared with placebo, but it was not different from oseltamivir (choice B is incorrect). The findings from a 2021 meta-analysis that included 3 randomized controlled trials (CAPSTONE-1, CAPSTONE-2, miniSTONE-2) that included 3,771 patients suggested that baloxavir is superior to placebo in the treatment of influenza in both clinical outcome and virological response. In addition, baloxavir was found to have a better virological response than oseltamivir and to be as effective as oseltamivir clinically with no statistically significant differences in the time to symptoms alleviation. The FLAGSTONE study published in 2022 showed that combining baloxavir with neuraminidase inhibitors (eg, oseltamivir, zanamivir, and peramivir, selected and administered according to local standard practice) did not result in superior clinical outcomes compared with neuraminidase inhibitors alone.
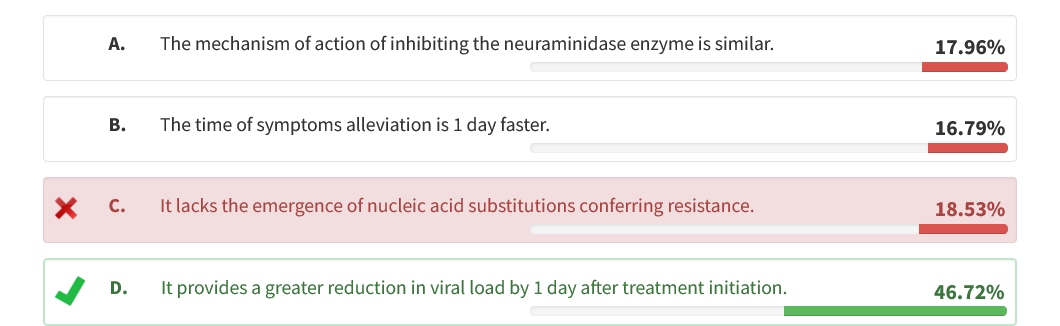
The emergence of nucleic acid substitutions conferring resistance to baloxavir occurred in 2% and 10% of recipients in both randomized controlled trials, respectively (choice C is incorrect). Baloxavir marboxil is a new antiviral treatment option for acute uncomplicated influenza infection in patients 12 years or older presenting within 48 h of symptom onset.1
Amantadine and rimantadine are not currently recommended by the United States Centers for Disease Control and Prevention for treatment of recent influenza strains owing to high rates of resistance, although recommendations vary annually based on resistance patterns of circulating strains. While time to clinical response is similar between IV zanamivir and oral oseltamivir, nebulized zanamivir is contraindicated in patients who are intubated owing to multiple reports of the drug causing filter obstruction of the ventilator leading to fatal hypoxemia. Because of the potential for bronchospasm, nebulized zanamivir is also not recommended for use in patients with underlying airway disease.