ESRD
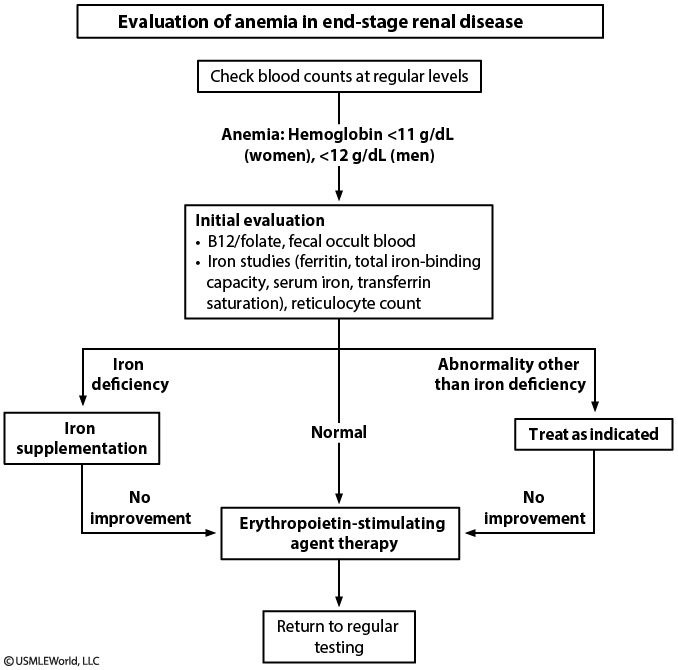
Causes
- ESRD anemia causes usually from decreased EPO
- Other causes:
- iron deficiency: These patients can develop iron deficiency due to blood loss from frequent blood testing, gastrointestinal blood loss (common in those with ESRD), or dialysis itself.
- severe hyperparathyroidism (which causes erythropoietin resistance)
- folate deficiency
- systemic inflammation
- aluminum toxicity
Criteria
- Absolute iron deficiency
- Absolute iron deficiency is defined as transferrin saturation <20% or ferritin <100 ng/mL.
- However, the underlying inflammation associated with ESRD and dialysis can significantly increase serum ferritin and make it a less accurate measure of iron deficiency.
- Functional iron deficiency
- Patients with ESRD can have functional iron deficiency (normal iron stores with inability to mobilize the stores in response to erythropoietin), defined as transferrin saturation <20% with ferritin of 100-800 ng/mL or higher.
- Because erythropoietic-stimulating agents (ESAs) increase iron demand, these patients should have iron studies prior to starting ESAs. Testing should continue periodically, especially in those without appropriate response to ESAs.
Treatment
- ESAs such as erythropoietin are recommended for ESRD patients with hemoglobin <10 g/dL. The goal is to increase hemoglobin by 1.5-2 g/dL over 4-6 weeks to target hemoglobin to 10-11.5 g/dL.
- Iron supplementation is recommended for ESRD patients with transferrin saturation <30% and ferritin <500 ng/mL. These patients can have higher iron requirements than they can absorb from the gastrointestinal tract and may not respond to oral iron. As a result, intravenous iron is suggested for patients on hemodialysis or peritoneal dialysis (oral iron is preferred for non-dialysis patients due to cost and convenience).
- Patients with ferritin >500 ng/mL (generally reflecting good iron stores) usually respond to increased ESA dose but may require intravenous iron if there is no response. This patient had an adequate initial response to ESA but now has recurrent anemia with low ferritin that suggests iron deficiency requiring treatment with intravenous iron.
The management of acute monoarticular gout is challenging in patients with renal failure or in those who have just undergone a renal transplant. Appropriate treatment options are the administration of intraarticular glucocorticoids or increasing the dosage of systemic glucocorticoids if the patient is on a lower dose. Since many patients receive glucocorticoids during the post-transplant period, they may respond to an increased dose of systemic glucocorticoids. However, the disadvantage with systemic glucocorticoids lies in their systemic side effects. For this reason, most rheumatologists prefer to use intraarticular glucocorticoids in the treatment of renal failure and post-transplant patients.
(Choice A) Nonsteroidal antiinflammatory agents (NSAIDs) can decrease renal prostaglandin production. As cyclosporin can also cause a decrease in the production of renal prostaglandin, the concomitant use of both drugs during the post-transplant period may cause significant reduction in renal blood flow and compromise the function of the transplanted kidney.
(Choice B) Allopurinol should never be used for treating acute monoarticular gouty arthritis. It may be used as a prophylactic agent to prevent recurrent attacks of gouty arthritis; however, once allopurinol is started, the dose of azathioprine should be reduced by 50-70% to prevent toxicity. Allopurinol decreases the activity of the enzyme xanthine oxidase that is responsible for the metabolism of azathioprine.
Probenecid is a uricosuric agent. It is not indicated for the treatment of acute gouty arthritis. Furthermore, this agent is not preferable in renal failure or post-transplant patients because it does not have any effect once the creatinine clearance decreases to less than 350 mg/min.
(Choice E) Colchicine can be used for acute gouty arthritis; however, concomitant use with azathioprine is associated with significant leukopenia.