explanatory vs pragmatic trials
- related: Biostats
- tags: #literature
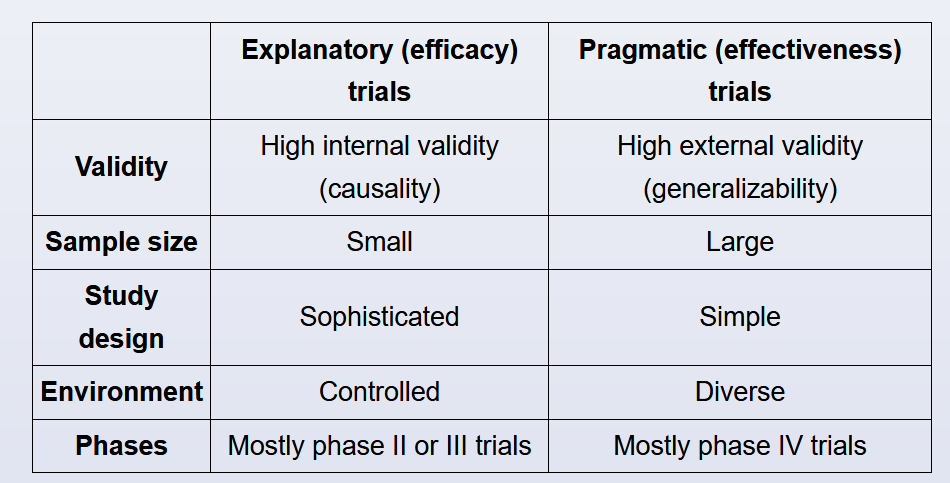
Clinical trials can be classified into 2 broad categories:
- Explanatory trials are designed to test the efficacy of interventions under optimal and controlled situations.
- Pragmatic trials are designed to evaluate the effectiveness of interventions in real-life (everyday) clinical practice conditions.
Pragmatic trials produce results that can be generalized and applied to the population on which the intervention was meant to be used. By contrast, results from explanatory trials often fail to be generalizable to the broader population.
In this example, physicians want to ensure the study population is as similar as possible to the population on which the intervention is meant to be used (ie, range of disease severity, comorbidities, age, sex, and social and ethnic diversity). Therefore, the proposed study is a pragmatic trial aimed at evaluating the effectiveness (as opposed to the efficacy) of treatment for patients with evidence of active Crohn disease.
Exploratory trials should be conducted early in phase I trials and involve limited human exposure. These types of design have no therapeutic or diagnostic intent and are not meant to examine the maximum tolerated dose. The aim is usually to prove a concept or to select a compound for full development, among many other possibilities.
(Choice E) A pragmatic study seeks to determine whether an intervention works in real-life conditions. This is contrasted with an explanatory study, which seeks to address whether an intervention works in optimal conditions and how/why it does or does not work. In general, randomized trials are considered explanatory, as randomization and other methods to control for confounders are not performed in the real world.