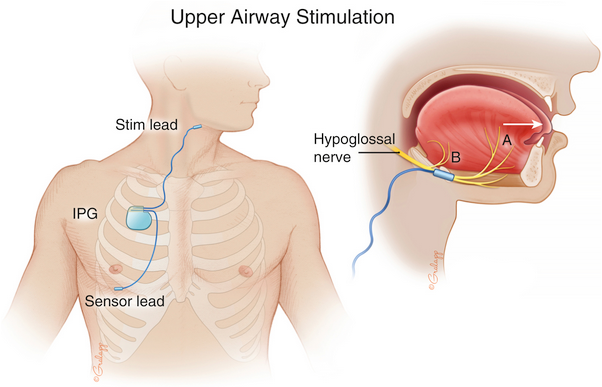
hypoglossal nerve stimulator
- related: Sleep and Sleep Disordered Breathing
- tags: #permanent
- INSPIRE device
- stimulates medial hypoglossal nerve and C1 nerve
- tongue stiffening and protrusion during sleep at end of expiration
- clinical indication:
- age > 18
- AHI 15-65
- can’t tolerate CPAP
- BMI < 35
- no concentric collapse with DISE
 1
1
Hypoglossal nerve stimulator (HNS) is a treatment option for patients with moderate to severe OSA who are unable to use PAP or do not derive benefit from PAP therapy. To qualify for HNS, the patient must be older than 18 years of age, have a diagnosis of moderate to severe OSA with AHI between 15 and 65 events per hour (of which less than 25% of events are central and mixed apneas), failure or intolerance of PAP therapy, BMI less than 35 kg/m2, and absence of complete concentric collapse at the level of velopharynx or soft palate on drug-induced sleep endoscopy (DISE).
Patients who are interested in HNS need to undergo drug-induced sedation endoscopy (DISE), typically performed by an otolaryngologist. DISE is performed under the supervision of an anesthesiologist with infusion of propofol to induce sleep. The goal of DISE is to ensure the patient has anteroposterior narrowing of the upper airway during sleep. This is important because patients who have concentric narrowing of the upper airway on DISE tend not to respond to HNS.
The HNS is an implantable system that includes a pulse generator that with each inspiration, generates an electrical impulse to cranial nerve XII (hypoglossal nerve). By stimulation of the hypoglossal nerve, that innervates the extrinsic and intrinsic muscles of the tongue, the tongue is moved forward with each inspiration, leading to an improvement in upper airway patency. This patient meets all the criteria, and if drug-induced sleep endoscopy confirms the absence of concentric collapse of velopharynx or soft palate, she would be an ideal candidate for HNS (choice B is correct). Approximately one-third of patients who undergo drug-induced sedation endoscopy have complete concentric collapse of the velopharynx and are therefore not candidates for hypoglossal nerve stimulator. Studies have shown long-term success in treating OSA with HNS in appropriately selected patients.
Serious adverse events are uncommon with HNS implantation (<2%). Some of these adverse events include tongue weakness, hematoma, surgical site infection, and postoperative pain. Most of these adverse events improve spontaneously over time or require conservative management. The battery typically lasts 10 to 11 years, after which the pulse generator will need to be replaced surgically. The newer generation of Inspire HNS (manufactured after summer 2022) is MRI compatible.
Although mandibular advancement devices can successfully treat mild and moderate OSA, their success rate is lower in patients with severe OSA. Additionally, this patient has temporomandibular joint discomfort and limited range of motion of the jaw, making an oral appliance not an ideal therapeutic alternative (choice A is incorrect). Her in-laboratory polysomnogram did not reveal any evidence of positionality, making positional therapy not an option (choice C is incorrect). Switching this patient from CPAP to BPAP is unlikely to resolve the problem because she is simply not interested in wearing a mask on her face (choice D is incorrect).
Weight loss is an important treatment strategy to decrease cardiometabolic risk in patients with OSA. Weight loss may also lead to improvement of OSA and could be considered in this patient if she were to not qualify for HNS owing to concentric collapse of the velopharynx on drug-induced sedation endoscopy. Weight loss of 17.64 to 22.05 lb (8 to 10 kg) can be achieved with intensive lifestyle interventions, but typically, lifestyle interventions do not lead to sustained weight loss. In addition to surgical weight loss interventions (ie, bariatric surgery), significant weight loss can be achieved with weekly subcutaneous injections of tirzepatide (a dual glucose-dependent insulinotropic polypeptide) or semaglutide (a glucagon-like peptide-1 receptor agonist). In a randomized, placebo-controlled trial, weekly subcutaneous tirzepatide at a dose of 15 mg led to 21% weight loss (48.5 lb [22 kg]) over 72 weeks of therapy. Although this clinical trial did not directly test the impact of weight loss on OSA, in most patients, 20% weight loss leads to an improvement in OSA severity. Moreover, such a degree of weight loss can also lead to additional cardiometabolic benefits.