initiating daytime ventilation for neuromuscular disease patients
- related: Sleep and Sleep Disordered Breathing
- tags: #literature #pulmonology
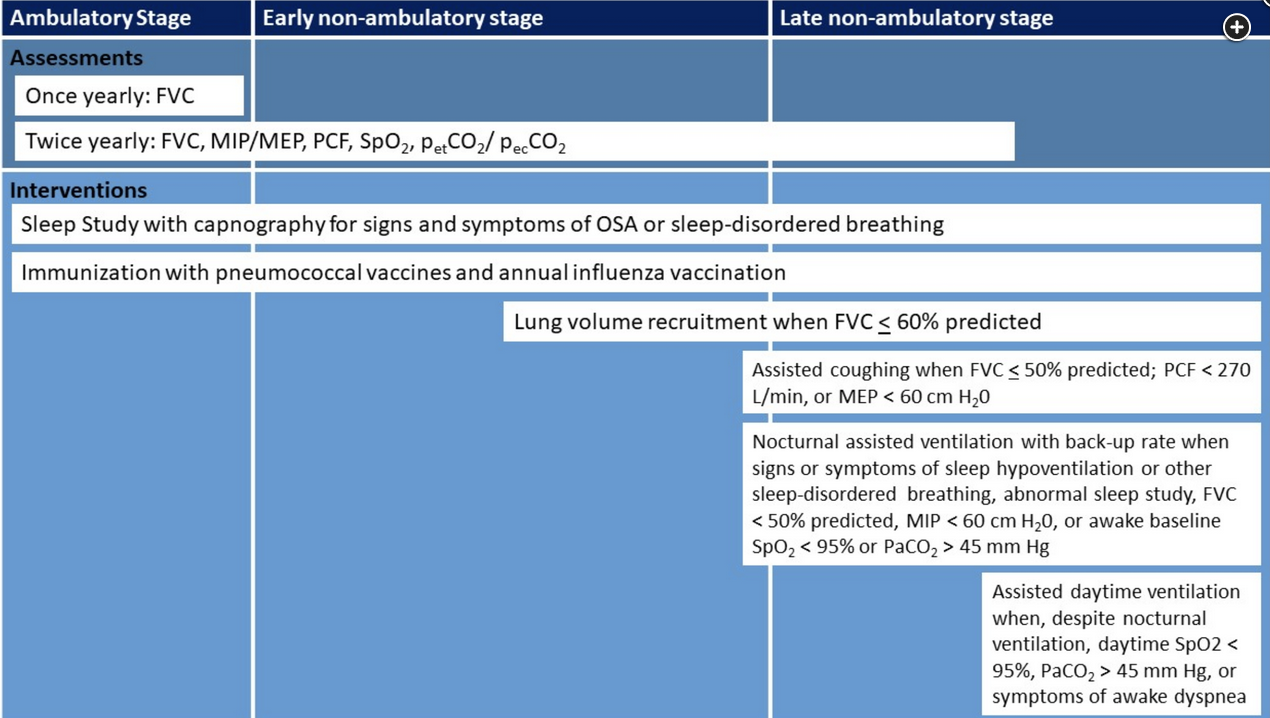
Given this patient’s history of DMD with associated neuromuscular weakness, restrictive physiology, and daytime hypercapnia—despite use of nocturnal assisted noninvasive ventilation—both nocturnal and daytime assisted noninvasive ventilation should be recommended (choice B is correct). DMD is a progressive, ultimately lethal neuromuscular disorder transmitted by X-linked inheritance. In DMD, mutations in the dystrophin gene cause an absence of dystrophin protein, harming the stability, strength, and functionality of myofibers and causing weakness in the skeletal muscles, including the muscles of respiration, in addition to cardiomyopathy. Pulmonary restriction worsens over time, with shallow breathing, ineffective coughing, and eventual stiffness of the chest wall, atelectasis, pneumonia, and respiratory failure. As a result, respiratory complications are a common cause of death, with a mean life span of around 20 years when the disease follows its natural course. Respiratory complications are a major cause of morbidity and mortality in patients with DMD. With declining pulmonary function, patients develop symptoms of hypoventilation such as dyspnea, fatigue, and difficulty concentrating, despite their use of assisted ventilation during sleep. Indications for extended use of assisted daytime ventilation include daytime SpO2 <95%, PaCO2 >45 mm Hg, or symptoms of awake dyspnea (Figure 1). This patient was already appropriately placed on nocturnal assisted noninvasive ventilation with a backup rate and despite this intervention had a daytime PaCO2 of 51 mm Hg (choice A is incorrect).
Formal measurement of respiratory muscle strength should be performed with maximum inspiratory pressure and maximum expiratory pressure (MEP) testing; either being lower than 60 cm H2O would also qualify him for NIPPV.
The core respiratory interventions for patients with DMD are lung volume recruitment, assisted coughing, nocturnally assisted ventilation, and subsequent daytime ventilation. During the early nonambulatory phase of the disease, lung volume recruitment using a self-inflating manual ventilation bag or mechanical insufflation-exsufflation to achieve deep lung inflation (when vital capacity is <60% predicted) is recommended. As these patients enter the late nonambulatory phase of the disease, manual and mechanically assisted cough techniques (when vital capacity is <50% predicted, peak cough flow is <270 L/min, or maximum expiratory pressure is <60 cm H2O) is recommended. In the late nonambulatory stage, individuals with DMD benefit from assisted ventilation to prolong survival. Ventilation devices should incorporate a backup rate of breathing to avoid apnea. Indications for nocturnal assisted ventilation include signs or symptoms of hypoventilation or sleep-disordered breathing, regardless of the level of pulmonary function; relevant symptoms include fatigue, dyspnea, morning or continuous headaches, frequent nocturnal awakenings or difficult arousal, hypersomnolence, difficulty concentrating, awakenings with dyspnea and tachycardia, and frequent nightmares. However, some patients remain asymptomatic despite the presence of hypoventilation. Thus, nocturnal assisted ventilation should be initiated when vital capacity is <50% predicted, or when the absolute value of maximum inspiratory pressure is <60 cm H2O, or when awake baseline SpO2 is <95% or PaCO2 >45 mm Hg (Figure 1).
Noninvasive methods of assisted ventilation instead of tracheostomy are favored to optimize patient quality of life. Consensus guidelines endorse the use of noninvasive ventilation, recognizing that clinical experience supports noninvasive assisted ventilation for up to 24 h a day. Additionally, observational evidence suggests that continuous noninvasive assisted ventilation can obviate the need for tracheostomy. Indications for tracheostomy include patient preference, inability of patient to use noninvasive ventilation successfully, three failed extubation attempts during a critical illness despite optimum use of noninvasive ventilation and mechanically assisted coughing, and failure of noninvasive methods of cough assistance to prevent aspiration of secretions into the lungs owing to weak bulbar muscles (choice C is incorrect).
Nocturnal supplement oxygen at 2 L/min would not improve the patient’s ventilation and may worsen his hypercapnia (choice D is incorrect).1
Maximal sniff inspiratory pressure (SNIP), which does not require mouthpiece for assessment, is an excellent method for following patients with neuromuscular disease with bulbar involvement and can guide the need for ventilatory support (choice D is correct).
ALS is a progressive disease with most patients dying within 3 to 5 years of diagnosis. Nonetheless, approximately 30% of patients survive 5 or more years, and 10% to 20% of patients survive more than 10 years after diagnosis. Progressive ventilatory failure is common; however, timely use of NIV has been associated with improved survival and quality of life and is included in guidelines concerning supportive treatments. NIV should be used in lieu of invasive ventilation whenever possible (choice C is incorrect).
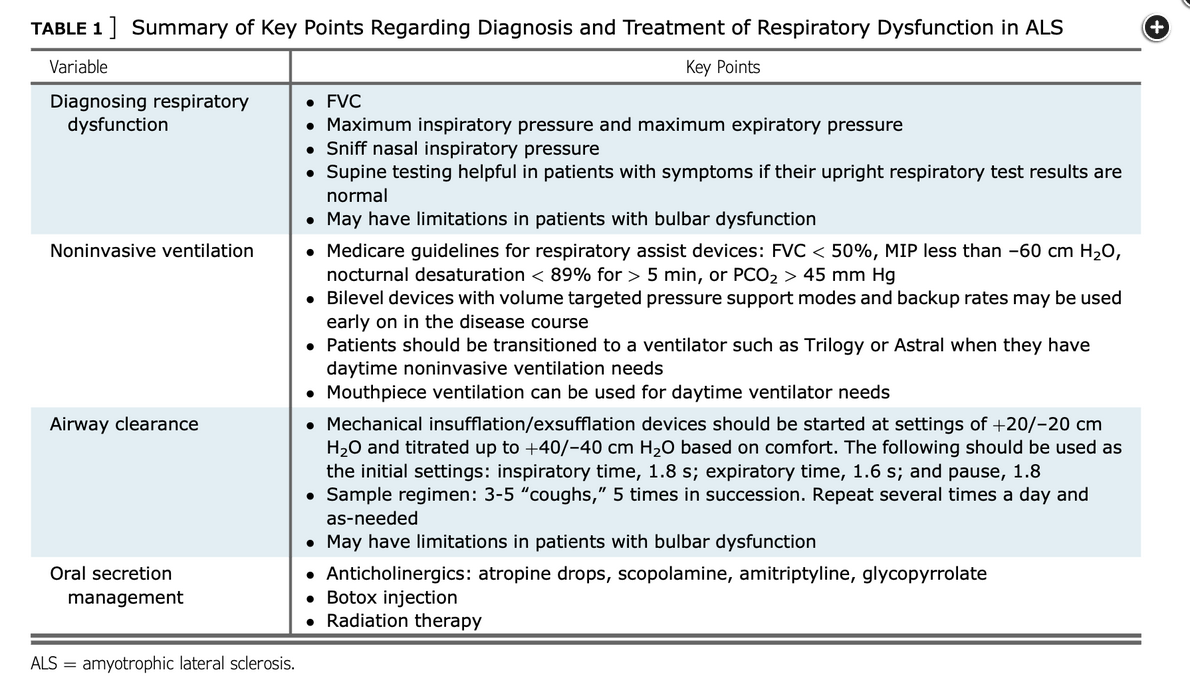
Recommended parameters to guide initiation of NIV that should be assessed frequently (usually at 3-month intervals) are VC less than 50% predicted, the presence of orthopnea, a maximal inspiratory pressure (MIP) less than −60 cm H2O, or abnormal nocturnal oximetry. Awaiting frank respiratory failure or the appearance of hypercapnia as determined by an arterial blood gas carries unnecessary risk. In fact, using tests that identify increasing risk of ventilatory failure in advance of blood gas abnormalities is the goal of prudent monitoring (choice B is incorrect).
Since patients with ALS may present with or develop bulbar weakness over time, performance of pulmonary function testing can be challenging. Inability to maintain a seal around the mouthpiece confounds performance of the VC and MIP maneuvers. VC and MIP may be erroneously interpreted as low because of the leak around the mouthpiece (choice A is incorrect).
An attractive alternative is the measurement of the maximal SNIP. This measurement places a manometer in an occluded nostril while the patient makes a sniff through the contralateral, nonoccluded nostril. Studies have shown that many patients with neuromuscular disease unable to perform tests with a mouthpiece can perform this test, and the specificity and sensitivity of the test compare favorably with VC and NIV. A SNIP less than 40 cm H2O is generally the threshold for recommending initiation of ventilatory support.1