lung volume reduction surgery LVRS
- related: COPD chronic obstructive pulmonary disease, Pulmonary Diseases
- tags: #pulmonology #literature #pulmonology
Lung Volume Reduction Therapy
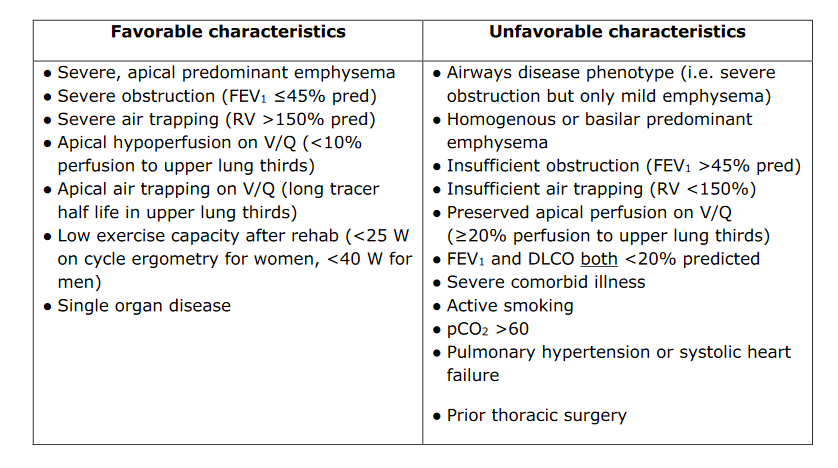
The National Emphysema Treatment Trial (NETT) demonstrated that patients with upper lobe–predominant and significant exercise limitations even after participation in a pulmonary rehabilitation program had improved quality of life, exercise tolerance, pulmonary function, and survival with lung volume reduction surgery. A key finding from NETT was that patient selection was critical to success and that patients with an FEV1 of less than 20% of predicted, a diffusing capacity of less than 20% of predicted, or non–upper lobe predominant disease had a high operative mortality and should not be offered lung volume reduction surgery. The procedure should be considered in patients with upper lobe–predominant who have low exercise tolerance despite maximal medical therapy and completion of a pulmonary rehabilitation program. Nonsurgical lung volume reduction procedures, including bronchoscopic placement of endobronchial one-way valves, plugs, or coils; administration of biologic sealants; and thermal ablation of the airway are being studied for treatment of emphysematous changes in patients with COPD. Early results suggest patients with severe air trapping and hyperinflation may get the most benefit from these new techniques.
The National Emphysema Treatment Trial is the largest randomized trial for lung volume reduction surgery and demonstrated a survival advantage after 6 weeks of pulmonary rehabilitation only in a high-risk subgroup of patients with predominant upper lobe emphysema and poor baseline exercise capacity.1