methemoglobinemia pathophysiology and causes
- related: methemoglobinemia has reduced O2 saturation with normal PaO2
- tags: #literature #icu #pulmonology
Methemoglobin is a form of hemoglobin in which 1 of the 4 iron molecules is oxidized to the ferric (Fe3+) rather than the normal ferrous (Fe2+) state. The ferric state has a decreased affinity for oxygen, but the remaining 3 ferrous heme sites have an increased oxygen affinity, which leads to decreased oxygen delivery to peripheral tissues.
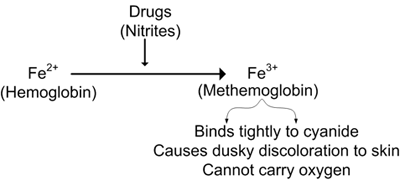
Methemoglobinemia is most commonly acquired after excessive exposure to an oxidizing agent. Many commonly used drugs are oxidants that may cause methemoglobinemia in susceptible patients. These include chloroquine, dapsone, trimethoprim, sulfonamides, local anesthetics (benzocaine, lidocaine), and nitrates (nitroglycerin, nitroprusside, nitric oxide).

Metoclopramide is used commonly to increase gastric motility. It is an oxidizing agent, converting ferrous iron (Fe++) in hemoglobin to the ferric form (Fe+++), creating MetHb. When given in excessive doses or to patients with deficiency in enzyme systems that convert methemoglobin to hemoglobin, toxic levels of MetHb may develop. MetHb has a much higher affinity for oxygen than oxyhemoglobin, and its presence reduces the oxygen content of arterial blood. The presence of MetHb also shifts the oxyhemoglobin curve to the left. Thus, both decreased oxygen content of arterial blood and increased affinity of oxygen for hemoglobin lead to reduced tissue oxygenation and the symptoms associated with methemoglobinemia.
The most likely diagnosis for this patient’s cyanosis and acute neurological decline is rasburicase-induced methemoglobinemia. Rasburicase is a recombinant form of urate oxidase approved for the prevention of hyperuricemia and acute uric acid nephropathy in patients at risk of tumor lysis syndrome. Hydrogen peroxide, a metabolic by-product of rasburicase, can drive the conversion of ferrous iron in hemoglobin to the ferric form in methemoglobin.