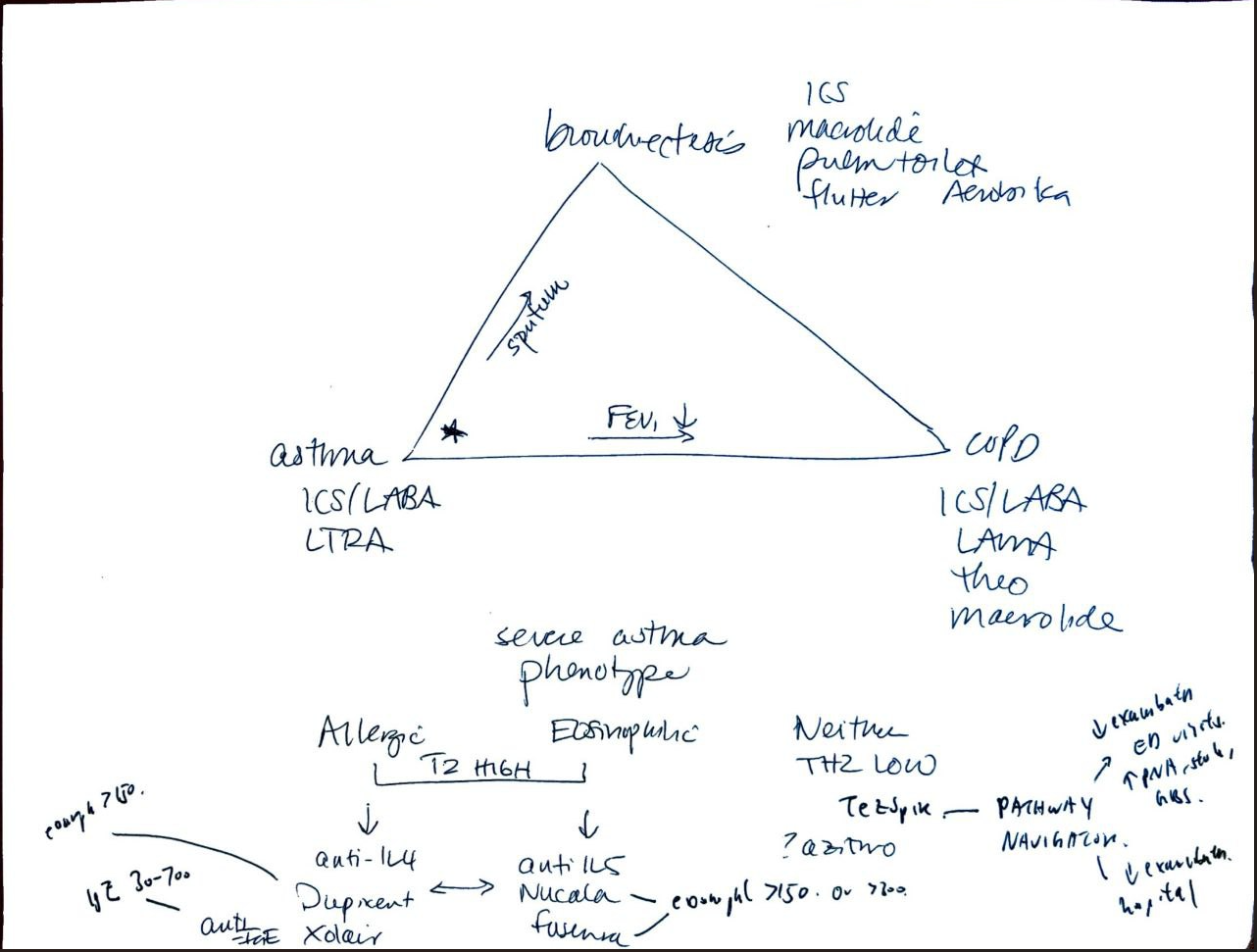
Moores triad of asthma management
- related: Asthma reactive airway disease
- tags: #literature #pulmonology
- Dupixent: dupilumab
- Xolair: omalizumab
- Nucala: mepolizumab
- Fasenra: benralizumab
- Tezspire: tezepelumab
- allergic asthma: xolair/dupixent
- eosinophilic asthma: nucala/fasenra
- neither: tezspire/macrolides
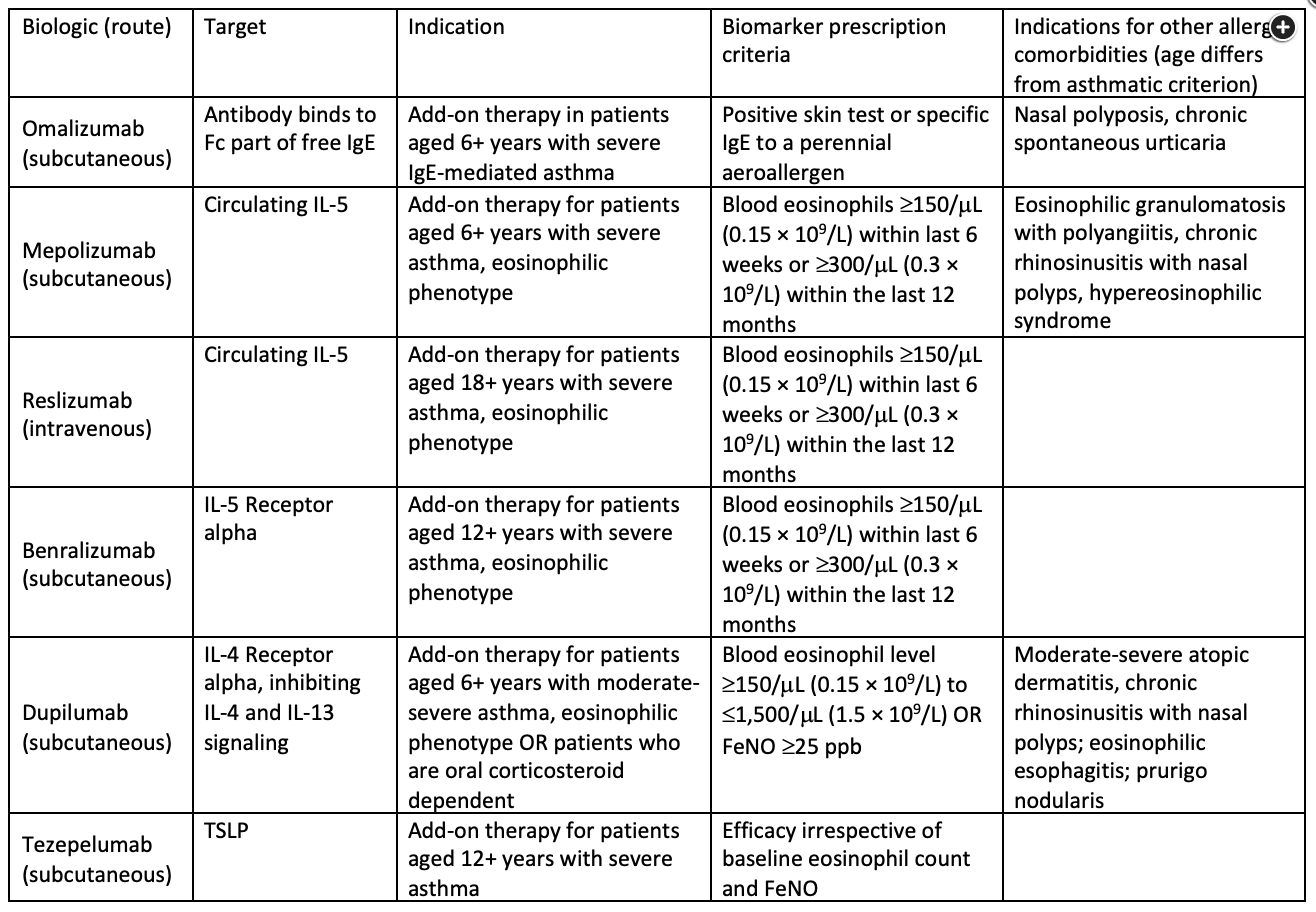 Abbreviations: TSLP, thymic stromal lymphopoietin; FeNO, fractional exhaled nitric oxide; ppb, parts per billion.
Abbreviations: TSLP, thymic stromal lymphopoietin; FeNO, fractional exhaled nitric oxide; ppb, parts per billion.
dupilumab dupixent is used to treat oral corticosteroid dependent asthma regardless of phenotype.
Omalizumab is a monoclonal antibody that binds to the Fc region of free IgE, preventing it from binding to receptors on mast cells, eosinophils, and basophils. This agent is used as an add-on to maintenance medications in patients whose asthma is not controlled with inhaled corticosteroids and who have evidence of allergic asthma on the basis of results from a positive skin-prick test or allergen-specific IgE (radioallergosorbent test) to a perennial aeroallergen and IgE count of 30 to 700 IU/mL (in patients over 12 years old).
Mepolizumab and benralizumab are monoclonal antibodies that target circulating IL-5 and the IL-5 receptor, respectively, both downstream effectors of allergic and eosinophilic inflammation. These agents, when added to maintenance therapy, can improve disease control and health-related quality of life for patients with poorly controlled asthma and an eosinophilic endotype (baseline pretreatment peripheral blood eosinophil level ≥150 cells/μL for mepolizumab and ≥400 cells/µL fo reslizumab).
Blood eosinophilia is a predictor of a therapeutic response. Tezepelumab, although not offered here, would have also been an option for this patient without evidence of T2 airway inflammation. This biologic agent targets thymic stromal lymphopoietin, which is released after various environmental insults to the airway epithelium. In a trial in patients with uncontrolled asthma who were receiving a mediumor high-dose inhaled corticosteroid plus at least one other controller, those who received tezepelumab had fewer exacerbations irrespective of baseline eosinophil count.