noninferiority trials
- related: Biostats and Study Design
- tags:
- source:
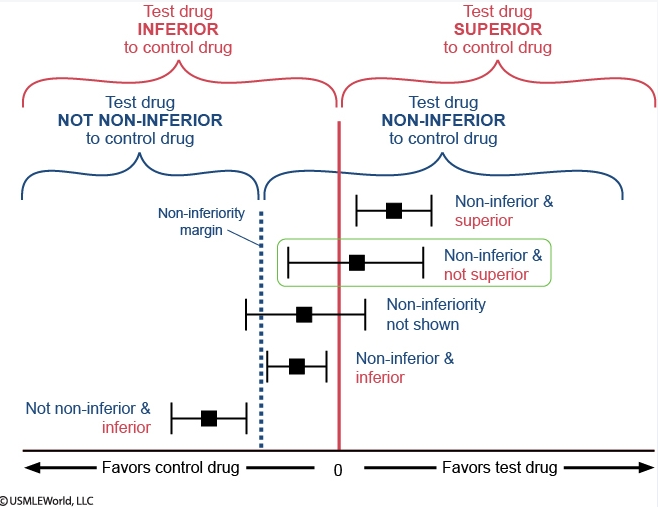
In the figure, each effect estimate for drug A is represented by a black square, with the horizontal black line representing the confidence interval.
The vertical red line centered at 0 splits the figure into 2 regions that compare drug A to warfarin: the region of superiority of drug A (“favors drug A”) lies to the right and the region of inferiority of drug A (“favors warfarin”) lies to the left. If the confidence interval crosses the red line, superiority cannot be proven. In superiority trials, the null hypothesis is that the effects of the drugs being compared are similar and the goal is to show that the new drug is different (better) than the comparator.
Similarly, the dotted blue line labeled “non-inferiority margin” also splits the figure into 2 regions that also compare drug A to warfarin: the region of non-inferiority of drug A lies to the right and the region of not non-inferiority of drug A lies to the left. In non-inferiority trials, the goal is to prove that a new drug is not unacceptably worse than a comparator (ie, the new drug should not be worse by more than an acceptable margin). The non-inferiority margin is used for this purpose and any effect estimate that lies entirely to its right is “non-inferior.” The opposite of “non-inferior” is “not non-inferior”; any effect estimate that lies entirely to the left of the non-inferiority margin is “not non-inferior” (unfortunately, the terminology is confusing and relies on the use of double negatives). If the confidence interval crosses the margin, this implies that non-inferiority is not proven. Non-inferiority should be assessed prior to superiority.
Regarding drug A and myocardial infarction, the effect estimate lies entirely to the right of the non-inferiority margin, so drug A is non-inferior to warfarin. The confidence interval crosses the vertical red line at 0, so it cannot be said that drug A is superior to warfarin. In sum, drug A is non-inferior and not superior to warfarin in preventing myocardial infarction.