post transplant pneumomediastinum can be from anastamotic dehiscence
- related: lung transplant and complications
- tags: #literature #icu
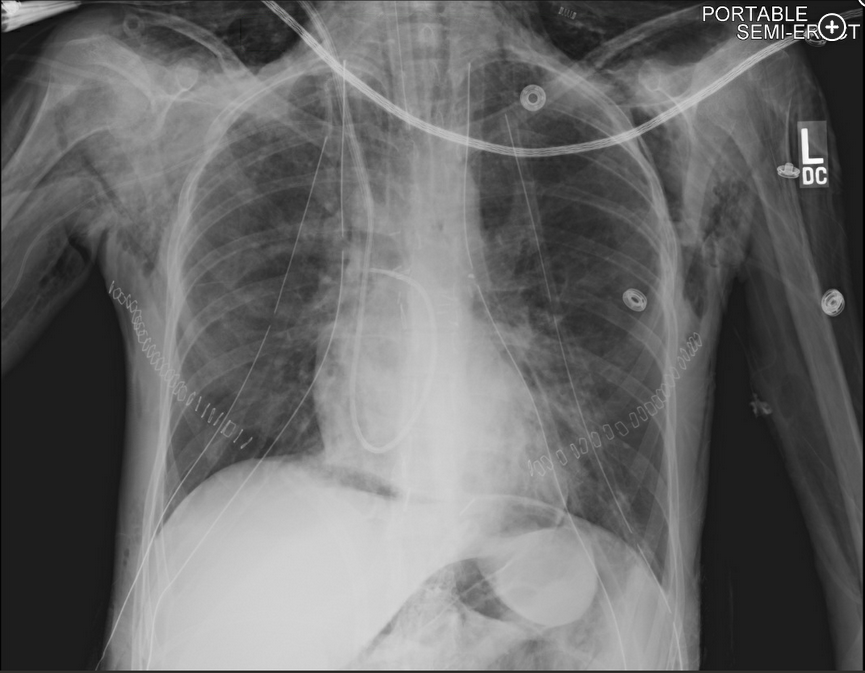
The chest radiography shows pneumomediastinum, which should suggest the possibility of anastamotic dehiscence. Pneumoperitoneum is also present.
Bronchoscopic visualization of the airway anastomosis should be performed to confirm the diagnosis, assess the severity of the defect, or exclude an anastomotic dehiscence. Dehiscence, the separation of the surgical anastomosis (Figure 2), is an infrequent complication after lung transplantation; however, it must be suspected when a lung transplant recipient develops a pneumothorax or pneumomediastinum in the early postoperative period.
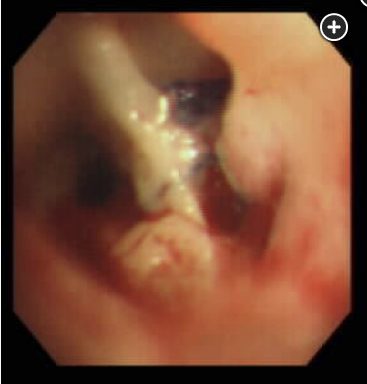
Airway complications occur in 7% to 18% of lung transplant recipients, with a related mortality of 2% to 4%. Airway complications are typically divided into early (<3 months) and late (>3 months) complications. Early airway complications include anastomotic infection, necrosis, or dehiscence. The incidence of anastomotic dehiscence ranges between 1% and 10%, with severe dehiscence reported in 2% of lung transplant recipients. Anastomotic dehiscence has been primarily attributed to ischemia of the donor bronchus in the first 1 to 6 weeks after transplantation. During lung transplant surgery, the airway anastomosis is performed between the bronchus of the donor lung and the bronchus of the recipient lung. Notably, the donor bronchial artery circulation is interrupted during the lung procurement procedure, and it is not routinely revascularized. As such, revascularization of the donor’s bronchus by the recipient’s bronchial arteries may take 2 to 4 weeks, placing the donor bronchus at risk for ischemia. Additionally, preoperative and postoperative pulmonary infections are significant risk factors for airway complications, and anastomotic infections increase the risk of dehiscence. Anastomotic dehiscence can present with dyspnea, pneumomediastinum, subcutaneous air, pneumothorax, persistent air leak, and the inability to wean from mechanical ventilation.
As management is dependent on the severity of the dehiscence, one must first assess the severity of the defect via direct bronchoscopic visualization prior to determining the best management strategy. Partial dehiscence is often treated conservatively with observation and close bronchoscopic surveillance, along with antimicrobial therapy. In cases of severe anastomotic dehiscence, deployment of temporary, self-expanding metal stents via bronchoscopy have been shown to facilitate healing. If bronchoscopic interventions fail, surgical interventions, such as open repair for reanastomosis, flap bronchoplasty, transplant pneumonectomy, or retransplantation may be considered. Anastomotic dehiscence is associated with a high mortality, and most patients die from infections and complications secondary to the dehiscence. His pneumoperitoneum is a result of his pneumomediastinum and should be managed conservatively.123456
Links to this note
Footnotes
-
Awori Hayanga JW, Aboagye JK, Shigemura N, et al. Airway complications after lung transplantation: contemporary survival and outcomes. J Heart Lung Transplant. 2016;35(10):1206-1211. PubMed ↩
-
Crespo MM, McCarthy DP, Hopkins PM, et al. ISHLT consensus statement on adult and pediatric airway complications after lung transplantation: definitions, grading system, and therapeutics. J Heart Lung Transplant. 2018;37(5):548-563. PubMed ↩
-
Machuzak M, Santacruz JF, Gildea T, et al. Airway complications after lung transplantation. Thorac Surg Clin. 2015;25(1):55-75. PubMed ↩
-
Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med. 2005;172(6):768-771. PubMed ↩
-
Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6(1):79-93. PubMed ↩