primary graft dysfunction PGD is acute noncardiogenic pulmonary edema from reperfusion injury
- related: lung transplant and complications
- tags: #literature #pulmonology
Primary graft dysfunction (PGD) is a form of noncardiogenic pulmonary edema that usually occurs within the first 72 hours post-transplantation. is likely caused by ischemia-reperfusion injury to the lung. It is a diagnosis of exclusion made clinically in the appropriate postoperative setting and is characterized by decreased gas exchange, a reduction in lung compliance, and the appearance of new alveolar infiltrates, often increased pulmonary vascular resistance, and intrapulmonary shunting..
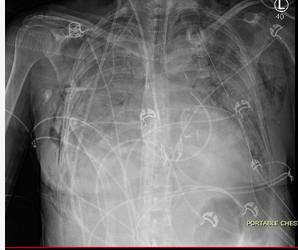
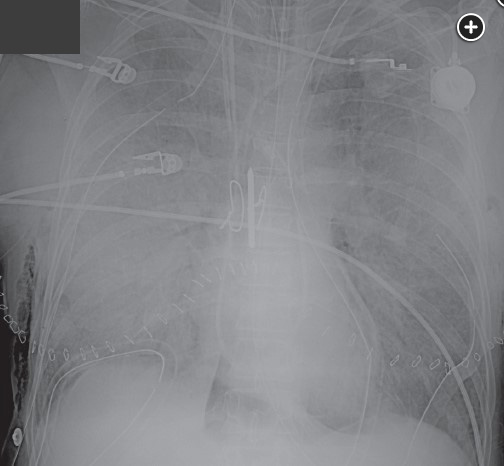
PGD was the subject of a four-part consensus statement discussing definition, causes, outcomes, risk factors, treatment, and prevention. There is a PGD Severity Grading system based on the impairment in gas exchange (based on the Pao2/Fio2 ratio) and the presence of infiltrates and is graded 0-3. The grading is conducted at times 0, 24 h, 48 h, and 72 h. Grade 0 is the absence of pulmonary edema on chest radiograph and Pao2/Fio2 >300, grades 1-3 all have pulmonary edema on chest radiograph and Pao2/Fio2 of >300, 200 to 300, and <200, respectively.
The incidence of PGD is about 30% for all grades combined and 15% to 20% for grade 3 PGD at 48 to 72 hours. PGD is assessed at the time of reperfusion of the graft, and at times 24, 48, and 72 hours following LT. The patient described in this case has PGD grade 3. Diffuse alveolar damage is the characteristic pathology, although it would be unusual to obtain pathology unless during autopsy.
Management of PGD includes supportive care with low tidal volume ventilation, empiric antibiotics, and sometimes inhaled nitric oxide (NO), inhaled prostaglandin, and ECMO (ideally started within 24 hours of the development of severe PGD). Retransplantation is associated with poor outcome and is avoided.1234567
A 45-year-old woman with pulmonary arterial hypertension in whom maximal medical therapy failed undergoes bilateral lung transplant from a donor after death by neurologic criteria. Her percentage of reactive antibodies was 48%, and the direct crossmatch revealed a good match. The patient was cytomegalovirus (CMV) negative, and the donor was CMV positive. The ischemic times were 287 min on the right and 349 min on the left. The off-pump surgery was uncomplicated, and 2 units of packed RBCs were transfused. In the operating room, a transesophageal echocardiogram revealed patent vascular anastomoses at the end of the surgery.
During transfer to the ICU, the patient was receiving low tidal volume ventilation with a PaO2/FIO2 (P/F) ratio of 400. Bilateral chest tubes were in place with no unusual bleeding. Bronchoscopy results revealed intact anastomoses, and bilateral bronchoalveolar lavage (BAL) fluid is sent for culture. A spontaneous breathing trial is successful, and the patient undergoes extubation 12 h postoperatively.
Twenty-four hours later, the patient now develops worsening oxygenation and requires reintubation. There was no witnessed aspiration before this event. There are new bilateral alveolar opacities on a chest radiograph (Figure 1). Vital signs are a temperature of 37.2 °C, pulse of 100/min, and BP of 120/70 mm Hg. SpO2 is 90% with FIO2 of 0.6 and PEEP is 8 cm H2O. Pulmonary artery occlusion pressure is 11 mm Hg. Arterial blood gas measurements are a pH of 7.38, PCO2 of 42 mm Hg, and PO2 of 70 mm Hg. Current medications include methylprednisolone, tacrolimus, mycophenolate mofetil, vancomycin, piperacillin-tazobactam, ganciclovir, posaconazole, and trimethoprim-sulfamethoxazole. There have been no additional blood transfusions. The brain-type natriuretic peptide (BNP) level is normal, and BAL cultures are negative to date. Results of a bedside transthoracic echocardiogram are unremarkable.
What is the most likely diagnosis?
Links to this note
Footnotes
-
Cantu E, Diamond JM, Cevasco M, et al. Contemporary trends in PGD incidence, outcomes, and therapies. J Heart Lung Transplant. 2022;41(12):1839-1849. PubMed ↩
-
Diamond JM, Arcasoy S, Kennedy CC, et al. Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, part II: epidemiology, risk factors, and outcomes-a 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1104-1113. PubMed ↩
-
Harano T, Ryan JP, Morrell MR, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation. ASAIO J. 2021;67(9):1071-1078. PubMed ↩
-
Natalini JG, Clausen ES. Critical care management of the lung transplant recipient. Clin Chest Med. 2023;44(1):105-119. PubMed ↩
-
Natalini JG, Diamond JM. Primary graft dysfunction. Semin Respir Crit Care Med. 2021;42(3):368-379. PubMed ↩
-
Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: definition and grading-a 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097-1103. PubMed ↩