pulmonary alveolar microlithiasis is accumulation of calcium phosphate crystals
- related: Pulmonary Diseases
- tags: #literature #pulmonology
source
This patient has pulmonary alveolar microlithiasis (PAM), a rare, autosomal recessive, calcific disorder characterized by the widespread intralveolar accumulation of minute calcium phosphate deposits (ie, microliths). There is no known treatment other than lung transplantation (choice B is correct). The disease is found worldwide with a higher prevalence in Turkey, Japan, Italy, China, India, and the United States. Familiality is frequent (noted in about one-third of cases), but the clinical course has nongenetic variability. The pathophysiology is based on mutations in the SLC34A2 gene, which encodes a type IIb sodium dependent phosphate cotransporter (NPT2b) in alveolar type 2 cells, leading to intraalveolar accumulation of phosphate that favors the formation of microliths. The phosphate transporter has an important role in inorganic phosphate homeostasis because type 2 alveolar cells are important in surfactant regulation and its degradation product, phospholipid. When dysfunctional, type 2 alveolar cells, are unable to clear phosphate from the alveolar space, resulting in formation of calcified round microliths. There is no associated calcium disorder in PAM (choice D is incorrect).
Most patients are diagnosed under the age of 50 and many in their late teens and twenties. The disease may be asymptomatic at first and may be discovered on chest imaging done for other reasons, and clinical-radiological dissociation is common. PAM progresses gradually over years and patients develop symptoms including dyspnea on exertion, nonproductive cough, chest pain, and desaturation in young adulthood and, eventually, respiratory insufficiency and failure by the fourth to fifth decades. Extrasystemic manifestations are rare.
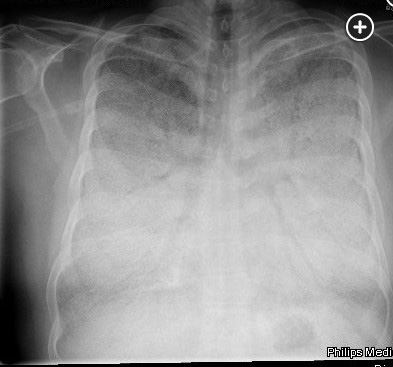
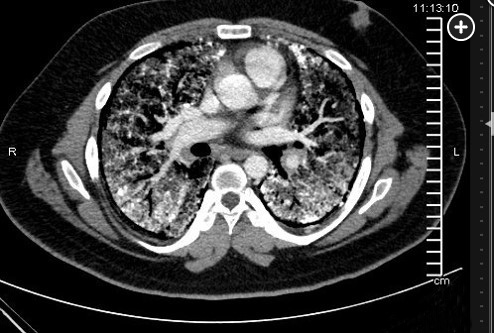
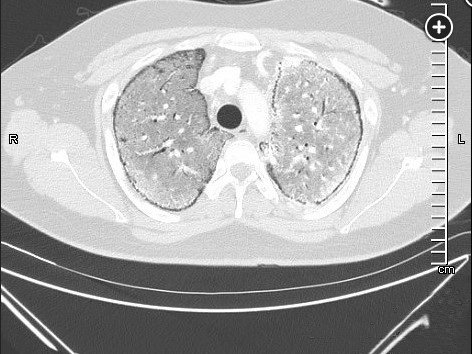
The diagnosis of PAM can be made with a high-resolution computed tomography (HRCT), perhaps with the addition of BAL, but in the appropriate familial setting, a chest radiograph may be enough. BAL may show lamellar microliths. Lung biopsy is rarely needed (choice A is incorrect). Labs are usually unremarkable, including serum calcium and phosphate levels. PFTs may be normal or show restriction with decreased diffusing capacity. The chest radiograph is typically described as having dense infiltrates in a “sandstorm” pattern of diffuse scattered micronodules (Figure 1) often with a lower lobe predominance. CT scanning shows micronodular calcification along the bronchovascular bundles and subpleural regions, and high density ground glass opacities (Figure 2, Figure 3). Treatment for PAM is supportive, including supplemental oxygen, and for those with advanced disease, lung transplantation is the only known effective therapy. There are case reports of treatment with corticosteroids, etidronate, chelating agents, and whole lung lavage without consistent success. Overall, untreated, the long-term prognosis is poor.
There are other causes of both ossification and calcification in the lungs. Metastatic pulmonary calcification is often asymptomatic with upper lobe predominant opacities and is associated with end stage renal disease requiring hemodialysis and secondary hyperparathyroidism, resulting in an increase in calcium and phosphate release from bone. Bone scanning may be helpful in the diagnosis and the treatment is aimed at the underlying disease. Renal transplantation may be effective. Dystrophic calcification may be seen with primary pulmonary amyloidosis due to light chain deposition in tissue, which appears in the airways and the parenchyma with confluent areas of subpleural calcifications in the mid and lower lung zones. Calcification can also be a late sequelae of a viral syndrome, such as varicella pneumonia, and typically appears as an asymptomatic miliary pattern of calcification (choice C is incorrect). Other infections, such as histoplasmosis and tuberculosis, can result in various patterns of calcification as well. Idiopathic pulmonary fibrosis can be associated with the development of <5 mm linear, peripheral, calcific densities known as dendriform pulmonary ossification (DPO), which are comprised of mature bone in the interstitial or alveolar spaces.