strep toxic shock syndrome can start from cellulitis
- related: necrotizing infections
- tags: #literature #id
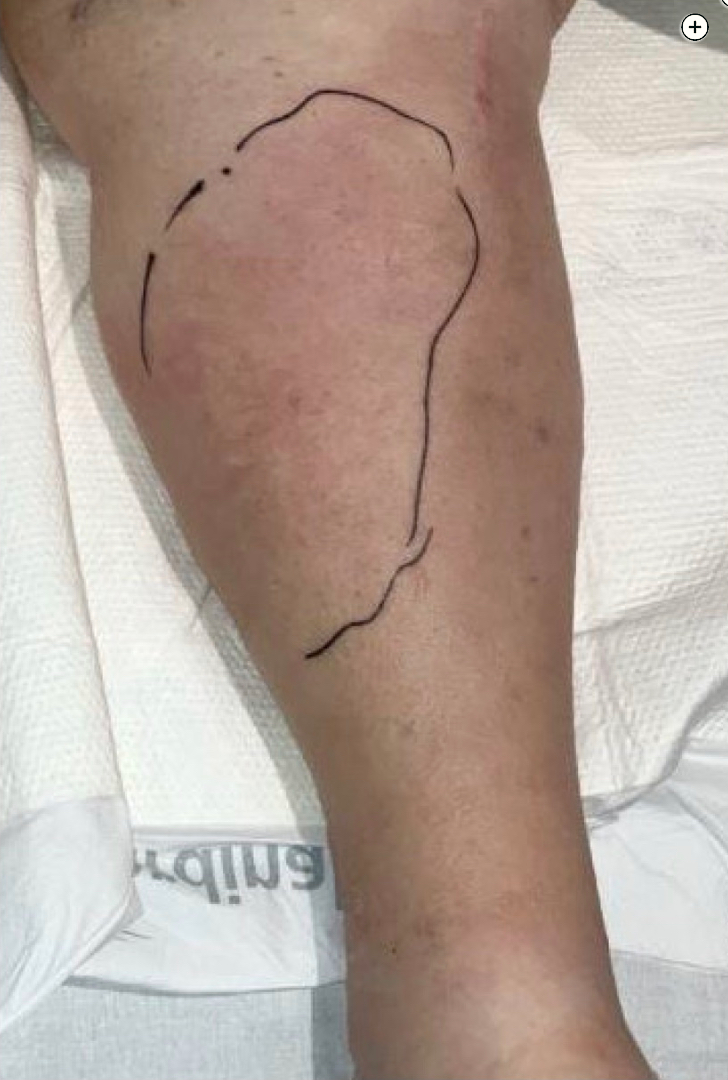
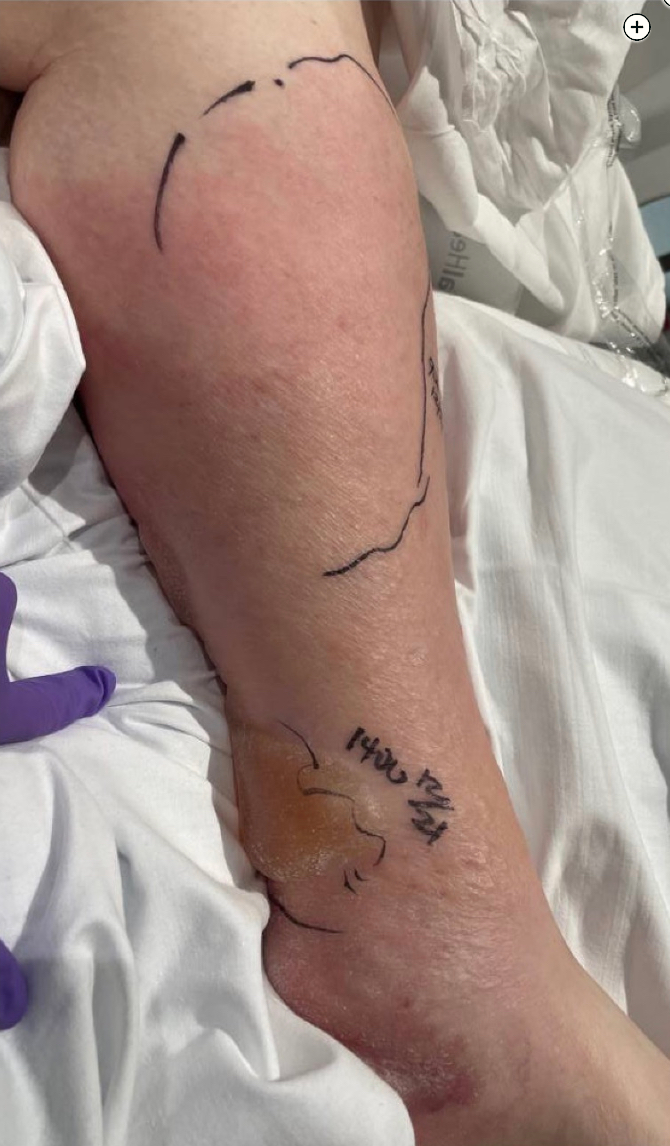
Skin and soft-tissue infections with invasive group A Streptococcus (GAS; S pyogenes) can be life-threatening and may be complicated by necrotizing soft-tissue infections (NSTIs) and/or toxic shock syndrome (TSS). Both NSTI and TSS are associated with high mortality rates (29% and 38%-59%, respectively). Streptococcal TSS (STSS) is an acute systemic illness that, if unrecognized and untreated, will rapidly progress to shock and multiorgan failure. It is essential for critical care physicians to recognize the early signs and symptoms of STSS; initiate treatment promptly, including appropriate antimicrobials and fluid resuscitation; and work closely with infectious disease experts and surgical teams because source control with surgical debridement is critical.
STSS most often originates from an invasive GAS skin or soft-tissue infection, such as cellulitis, which may then rapidly progress to necrotizing fasciitis. Invasive GAS produces a superantigen exotoxin that results in the release of proinflammatory cytokines, vasodilating mediators causing a capillary leak syndrome leading to shock and multiorgan failure.
Despite the fact that S pyogenes is usually extremely susceptible to penicillin, penicillin monotherapy is associated with increased morbidity and mortality in the setting of GAS infections with exotoxin production as occurs in STSS and NSTI. Several mechanisms contribute to the inadequacy of penicillin monotherapy, including the following: (1) exotoxin production, (2) the Eagle effect (a paradoxical decrease in penicillin killing activity with high bacterial inocula), and (3) penicillin-binding proteins being available only on the organism’s surface at specific phases of bacterial growth. Notably, clindamycin inhibits protein synthesis and thus streptococcal exotoxin production, which halts the ongoing stimulation of the systemic inflammatory cascade associated with TSS. Unlike penicillin, clindamycin efficacy is not affected by inoculum size or bacterial growth phase. For these reasons, combination therapy with penicillin plus clindamycin is the preferred antibiotic regimen for the treatment of NSTI and TSS invasive GAS infections that are susceptible to clindamycin.
The US Centers for Disease Control and Prevention’s active bacterial core surveillance reports that clindamycin resistance (inducible and constitutive) in GAS isolates nearly doubled from 2016 to 2020 (14.7% to 29.1%). Linezolid is an alternative agent that also inhibits protein synthesis and suppresses toxin production, and susceptibility of invasive GAS to linezolid remains high, with 100% susceptibility reported in nearly 4,000 US GAS clinical isolates between 2013 and 2020. Patients treated with linezolid also have lower incidence of subsequent Clostridium difficile infections. A retrospective single-center cohort study did not, however, find a difference in inpatient mortality, reduction of Sequential Organ Failure Assessment score over the first 72 h, or secondary outcomes (duration of vasopressor therapy, ICU length of stay) among patients receiving clindamycin (n = 26) and those receiving linezolid (n = 29) for severe GAS infections.
This patient has TSS due to GAS that is resistant to clindamycin. In this case, the most appropriate antibiotic therapy would be penicillin G plus linezolid. Initial empiric treatment of suspected TSS should include broad-spectrum antibiotics that cover both staphylococcal TSS and STSS. Once the diagnosis of STSS is established, treatment should be narrowed to penicillin G (4 million units intravenously every 4 h). Ceftriaxone or cefazolin are alternate antibiotics for patients with β-lactam hypersensitivity, and vancomycin should be used for patients with anaphylaxis to β-lactams. Neither vancomycin nor rifampin suppresses GAS toxin production, and there is no role for rifampin in the treatment of her skin infection and bacteremia. If one were to invoke a concern for polymicrobial sepsis (GAS and methicillin-resistant Staphylococcus aureus [MRSA]) from her skin infection, linezolid would treat MRSA and suppress GAS toxin production.123456
A 79-year-old patient with morbid obesity and type 2 diabetes mellitus presents with fever and left lower leg swelling and pain. She states she bumped her leg on her coffee table 2 days before presentation. She has a drug allergy to clindamycin. Her vital signs include a temperature of 39.2 °C, heart rate of 159/min, BP of 76/36 mm Hg, respiratory rate of 30/min, and SpO2 of 96% with 6-L nasal cannula.
Her examination results are notable for left lower extremity warmth, erythema, and diffuse tenderness to palpation that has worsened over the last several hours and now has blistering without crepitus (Figures 1 and 2) Her laboratory test results are notable for a WBC count of 27,000/μL (27 ×109/L) with 24% bands, creatinine level of 2.6 mg/dL (229.84 µmol/L) (up from baseline of 1.1 mg/dL [97.24 µmol/L]), lactate level of 40.54 mg/dL (4.5 mmol/L), and creatine kinase level of 800 U/L (13.36 μkat/L).
She receives 4 L of balanced fluids and requires norepinephrine at 8 μg/kg/min to maintain a mean arterial pressure greater than 65 mm Hg. She is treated empirically with vancomycin and piperacillin-tazobactam. Ten hours into her hospitalization, two out of two blood cultures are positive for Streptococcus pyogenes, which is susceptible to β-lactams but resistant to clindamycin. The surgical team has been called and is evaluating the patient. The team decides to change the antibiotic regimen.
What is the most appropriate antibiotic therapy?
Links to this note
Footnotes
-
Babiker A, Li X, Lai YL, et al. Effectiveness of adjunctive clindamycin in β-lactam antibiotic-treated patients with invasive β-haemolytic streptococcal infections in US hospitals: a retrospective multicentre cohort study. Lancet Infect Dis. 2021;21(5):697-710. PubMed ↩
-
Brown KA, Khanafer N, Daneman N, et al. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57(5):2326-2332. PubMed ↩
-
Cortés-Penfield N, Ryder JH. Should linezolid replace clindamycin as the adjunctive antimicrobial of choice in group A streptococcal necrotizing soft tissue infection and toxic shock syndrome? A focused debate. Clin Infect Dis. 2023;76(2):346-350. PubMed ↩
-
Heil EL, Kaur H, Atalla A, et al. Comparison of adjuvant clindamycin vs linezolid for severe invasive group A Streptococcus skin and soft tissue infections. Open Forum Infect Dis. 2023;10(12):ofad588. PubMed ↩
-
White BP, Siegrist EA. Increasing clindamycin resistance in group A streptococcus. Lancet Infect Dis. 2021;21(9):1208-1209. PubMed ↩