use trendelenburg position for air embolism from central line placement
- related: ICU intensive care unit
- tags: #literature #icu
The CT pulmonary angiogram demonstrates a large volume of air in the right atrium, ventricle, and pulmonary outflow tract, with leftward bowing of the interventricular septum, suggestive of acute right heart strain (Figure 1, Figure 2, Figure 3, Figure 4).
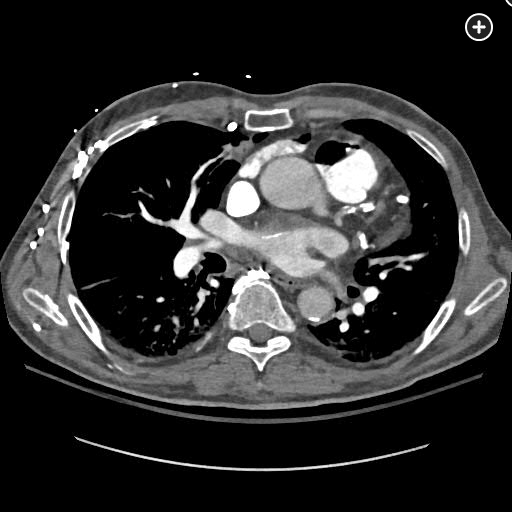
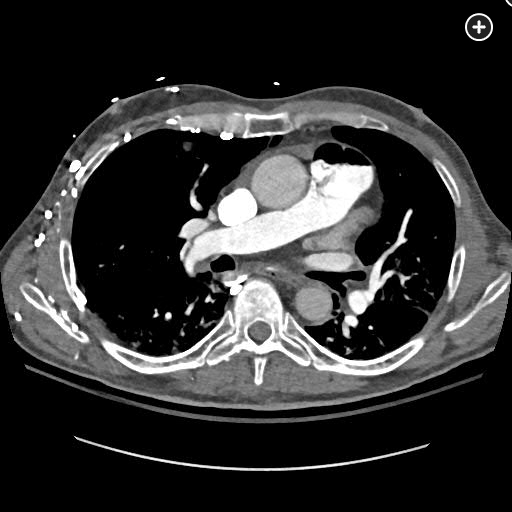
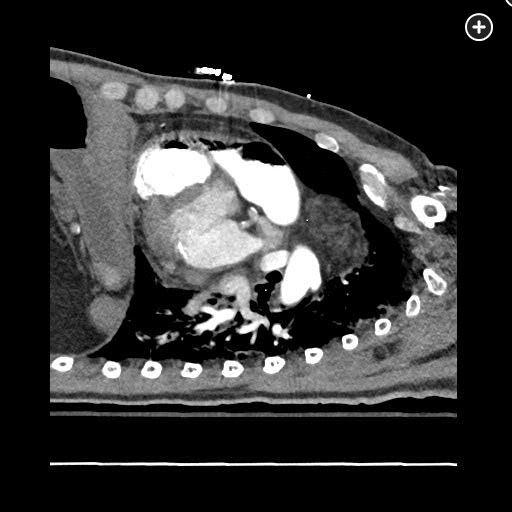
Sagittal CT image better demonstrating a massive air embolism involving the right ventricle and pulmonary outflow tract.
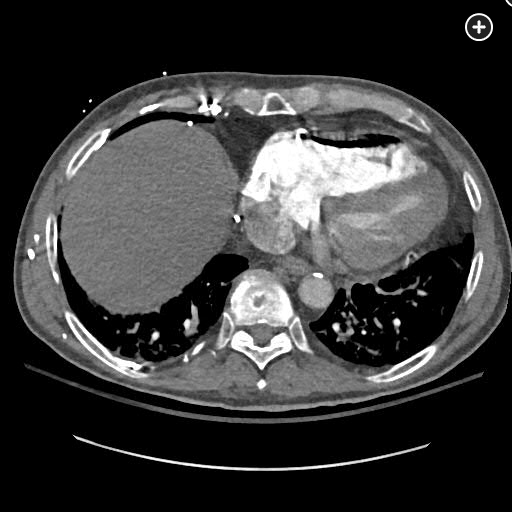
CT image demonstrating air in the right ventricle with leftward bowing of the interventricular septum, indicative of acute right heart strain.
Management priorities in this setting include emergent stabilization and identification and correction of the source of air entry, followed by reduction of the entrained gas volume. The most appropriate next management step is to place the patient in the left lateral decubitus (Durant’s maneuver) and Trendelenburg position, which positions the right ventricular outflow tract lower than the right ventricular cavity and encourages intravascular gas to migrate to the right ventricular apex. This reduces the risk of further right ventricular outflow tract obstruction and systemic air embolization.
Vascular air embolism (VAE) occurs when air or medical gas enters a patient’s venous or arterial circulation. The most common causes of VAE are medical interventions, including air entrainment through IV or central lines, endovascular or interventional procedures, surgery, trauma, labor and delivery, interventional pulmonary procedures, or mechanical ventilation. Cases associated with contrast injection, laparoscopic abdominal inflation, or intraaortic balloon rupture have been reported. VAE can also occur when scuba divers ascend too rapidly, causing alveolar rupture and arterial gas embolism.
Venous VAE events are most common due to vascular entrainment when an IV catheter system is not tightly closed, IV tubing is compromised, or residual air remains within the closed system. In this case, the source was one of the central venous catheter hubs, which had loosened during flight from helicopter vibrations. Small VAEs are typically absorbed in the pulmonary capillary bed and resolve without symptoms or with minor pulmonary complaints (coughing). Larger volumes of venous air cause pulmonary vasoconstriction, increased pulmonary artery pressure, elevated resistance to right ventricular outflow, and right ventricular failure. These physiological changes can also cause shifting of the interventricular septum, leading to decreased left ventricular filling, decreased cardiac output, and shock. Neutrophil activation in the pulmonary capillary bed can cause thromboxaneand leukotriene-mediated increases in airway resistance and pulmonary capillary permeability, resulting in pulmonary edema. In addition to the physical exam findings described in this case, a “mill-wheel” murmur may be present on cardiac exam. In severe cases, a large bolus of air can completely fill the right atrium and ventricular outflow tract, causing complete right ventricular outflow tract obstruction, arrhythmias, and cardiovascular collapse. Lethal volumes of air in humans have been estimated at 3 to 5 mL/kg or 100 to 300 mL injected rapidly.
Venous air can enter the arterial circulation as a paradoxical embolism, either when the absorptive capacity of the pulmonary capillary bed is overwhelmed or when an intrapulmonary (arteriovenous malformation) or intracardiac (patent foramen ovale, atrial septal defect) shunt is present. These air emboli can travel throughout the systemic circulation, with the most serious complications including cerebral embolization, stroke, and death. Systemic air also disrupts the microcirculation, triggering the release of proinflammatory and prothrombotic mediators, diffuse endovascular injury, microvascular thrombosis, and organ ischemia. Although direct arterial embolization has classically been associated with cardiopulmonary bypass, a large systematic review revealed that device malfunction during endovascular procedures accounted for 24% of these events, including steerable catheters, catheter introducers, angiographic injectors and syringes, and cardiac ablation percutaneous catheters.
Immediate management of venous VAE includes terminating the source of air, administering high-flow oxygen, and attempting to aspirate air from a central venous catheter. Left lateral decubitus and Trendelenburg positioning can prevent and acutely mitigate shock symptoms due to right ventricular outflow tract obstruction, and extracorporeal hemodynamic support may be required in severe cases. Patients with arterial air embolism should be placed flat to reduce the risk of cerebral gas embolism. Hyperbaric oxygen (HBO) is the primary therapy for these patients and is most effective if started within 4 to 6 h of symptom onset. The volume of air and hemodynamic instability in this case make the benefits of HBO without further stabilization questionable. Lidocaine administration may be considered to reduce the risk of seizures from cerebral air embolism (not present in this case). Systemic thrombolysis may be considered in the setting of massive pulmonary embolism, but the CT imaging in this case is not consistent with this diagnosis.12345
Links to this note
Footnotes
-
Brull SJ, Prielipp RC. Vascular air embolism: a silent hazard to patient safety. J Crit Care. 2017;42:255-263. PubMed ↩
-
Malik N, Claus PL, Illman JE, et al. Air embolism: diagnosis and management. Future Cardiol. 2017;13(4):365-378. PubMed ↩
-
McCarthy CJ, Behravesh S, Naidu SG, et al. Air embolism: diagnosis, clinical management and outcomes. Diagnostics (Basel). 2017;7(1):5. PubMed ↩
-
Toung TJ, Rossberg MI, Hutchins GM. Volume of air in a lethal venous air embolism. Anesthesiology. 2001;94(2):360-361. PubMed ↩