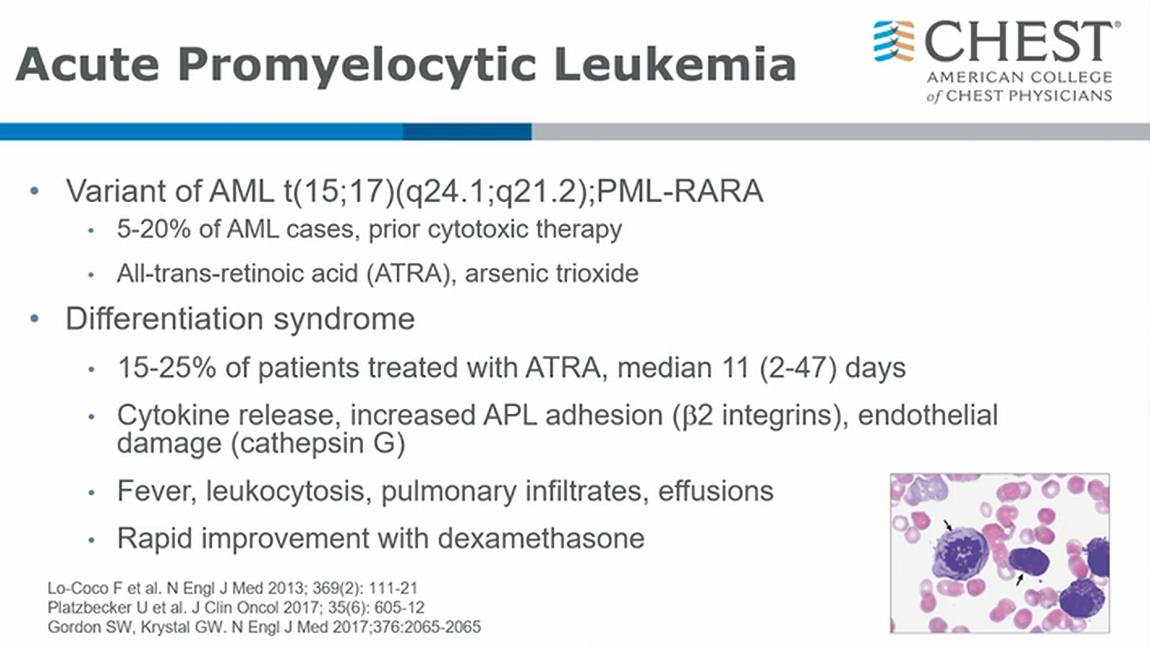
APML and differentiation syndrome aka retinoic acid syndrome
- related: Oncology
- tags: #literature #pulmonology
APML
APL, a subtype of acute myeloid leukemia (AML), is characterized by a distinctive morphology of blast cells, life-threatening coagulopathy, and a t(15;17) translocation—rearrangement of the PML and RARA genes (PML-RARA). APL, once a fatal disease, is now the most curable subtype of adult AML; however, the fatality rates remain high early in the disease. Early fatality has been attributed to spontaneous hemorrhage in the setting of severe coagulopathy or treatment-related complications.
- look for Aeur Rods
- APML often prior treatment related leukemia
- ATRA is treatment for good functioning pts, and arsenic for less good performant status patients1
Differentiation Syndrome
- develops with treatment of ATRA (all trans retinoic acid)
- ATRA help promyelocytes differentiate. However, as they differentiate, there is inflammatory reaction 1 - 1.5 weeks later. It’s essentially systemic inflammatory syndrome hard to distinguish from sepsis or heart failure
- once ruled out other causes, treat with dexamethasone1
Regimens that combine all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) are responsible for the marked improvement in APL fatality rates, but these regimens also cause differentiation syndrome (DS). APL DS is a relatively common life-threatening complication in the treatment of APL, often occurring within the first few weeks of treatment, that can occur when leukemic cells are induced to differentiate into more mature forms by ATRA or ATO. The signs and symptoms of APL DS are nonspecific. There are no universally accepted diagnostic criteria; however, one commonly used system includes seven elements and grades the severity of APL DS from indeterminate to severe. The patient has six of the seven features of APL DS, which correlates with severe APL DS. The standard of care is promptly starting dexamethasone 10 mg every 12 h when the clinician first suspects APL DS, even if in the indeterminate range. This recommendation is informed by multiple prospective and retrospective studies that suggest that the immediate initiation of steroids leads to a marked reduction in APL DS-associated mortality. For critical care physicians, it is essential to know the clinical presentation of APL and to recognize the early signs and symptoms of APL DS.
The pathogenesis of APL DS is complex and not well understood. ATRA induces terminal cell differentiation, which results in the differentiation of promyelocytic cells to mature cells. ATRA is believed to result in APL DS through multiple mechanistic pathways, including cytokine release causing systemic inflammatory response syndrome, increased vascular permeability, and endothelial damage, which is further precipitated by adhesion of blast cells causing leukostasis.
Early diagnosis of APL DS can be challenging because the signs and symptoms are nonspecific. The most common presenting signs and symptoms of APL DS include dyspnea, fever, weight gain of more than 5 kg, hypotension, acute kidney injury, and abnormal chest radiographs with the presence of pulmonary opacities or pleural effusions. Pericardial effusions are commonly encountered in APL DS. Most common abnormal laboratory findings of APL DS include leukocytosis (WBC count >10,000/μL [10 × 109/L]) and coagulopathy (prolongation of prothrombin time and partial thromboplastin time and decreased fibrinogen).
In parallel with starting dexamethasone 10 mg every 12 h, investigation for alternative causes should also be initiated, such as bacterial or fungal infections, sepsis, pneumonia, heart failure, or diffuse alveolar hemorrhage, because these have similar clinical manifestations and may occur concurrently with APL DS. ATRA and ATO should be held in the setting of severe DS, organ failure, and need for ICU admission. Evaluation for possible fungal infection is indicated, but empiric voriconazole would not be the best next step. In this patient with rapid onset of anuric renal failure in the setting of TLS, initiation of continuous renal replacement therapy would be indicated.2345
This patient has developed differentiation syndrome (formerly called retinoic acid syndrome). The syndrome occurs in approximately 25% of patients with acute promyelocytic leukemia during induction therapy that includes the differentiation agents all-trans retinoic acid (tretinoin) or arsenic trioxide and is believed to result from a release of inflammatory cytokines from malignant promyelocytes. No specific risk factors permit identification of patients with acute promyelocytic leukemia who are likely to develop differentiation syndrome.
The syndrome can include fever, peripheral edema with weight gain, pulmonary infiltrates, hypoxemia, respiratory distress, hypotension, renal and hepatic dysfunction, rash, and pleural and pericardial effusions. Arsenic trioxide also is associated with QT prolongation, although the patient in this scenario did not develop this complication.
Most antineoplastic medications have multiple adverse effects but are not associated with differentiation syndrome. Cytarabine causes bone marrow suppression and may cause cerebellar ataxia in high doses. Daunorubicin and other anthracyclines are best known for their potential cardiotoxicity and promotion of secondary hematologic malignancies. Etoposide is associated with bone marrow suppression, nausea and vomiting, and also can promote secondary malignancies.6
A previously healthy 27-year-old man presented with 1 week of bruising and bleeding from his gums. At admission, his laboratory test results were notable for mild anemia, thrombocytopenia, hypofibrinogenemia, and a WBC count of 9,000/μL (9 × 109/L) with 60% blasts and Auer rods on peripheral smear.
On hospital day 1, all-trans retinoic acid (ATRA) induction therapy is started for presumed acute promyelocytic leukemia (APL); allopurinol and intravascular fluids are started for tumor lysis syndrome (TLS) prophylaxis, along with piperacillin-tazobactam; and prophylaxis is started with micafungin and valacyclovir. The diagnosis of APL is confirmed with fluorescence in situ hybridization analysis, which is positive for t(15;17)(q24;q21) in 90% of cells, and his bone marrow biopsy result shows 68% blasts. Hydroxyurea is started for a climbing WBC count, and, on hospital day 4, arsenic trioxide (ATO) is started.
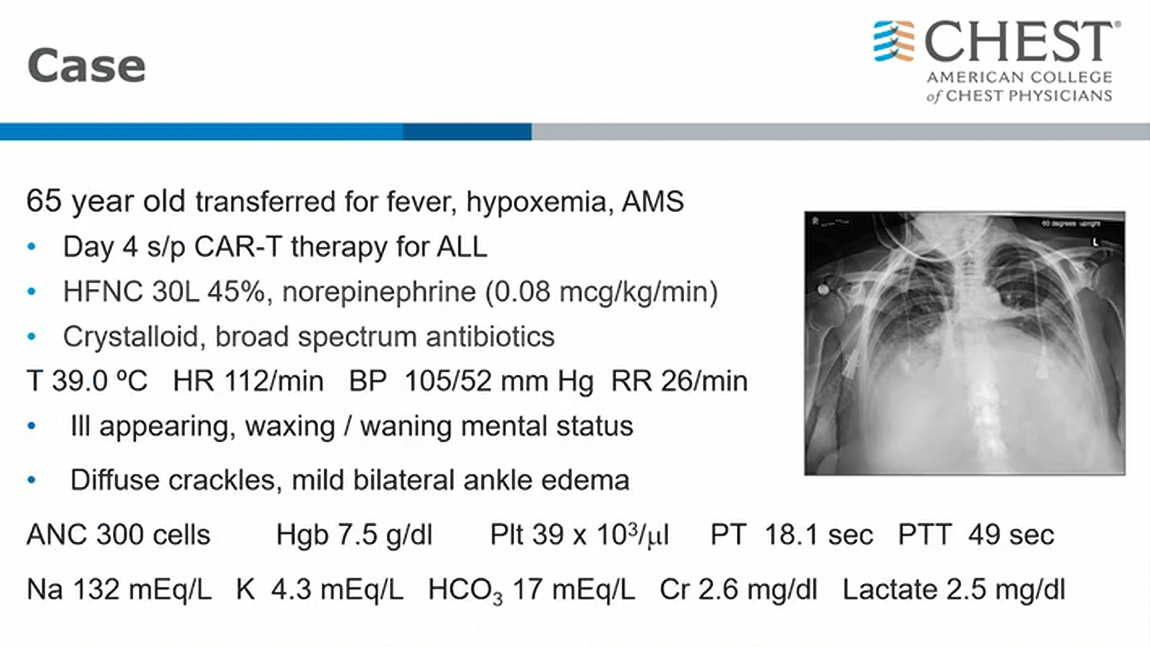
Two days later (hospital day 7), he develops myalgias, cough, dyspnea, acute hypoxemia, anuria, and worsening leukocytosis requiring transfer to the medical ICU. His vital signs include a temperature of 38.4 °C, heart rate of 128/min, BP of 80/52 mm Hg, respiratory rate of 28/min, 94% with high-flow nasal cannula (50 L/min, FIO2 of 0.6), and his weight is up 7 kg from his baseline weight. His lungs have crackles bilaterally, his cardiac examination results indicate he is tachycardic, and his extremities are warm with 2+ peripheral edema. Notable laboratory test results are shown in Figure 1, and his chest radiographs are shown in Figure 2. Results from his transthoracic echocardiogram are unremarkable.
What is the best next step in the treatment of the patient?
Links to this note
Footnotes
-
Iland HJ, Bradstock K, Supple SG, et al; Australasian Leukaemia and Lymphoma Group. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570-1580. PubMed ↩
-
Lo-Coco F, Avvisati G, Vignetti M, et al; Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111-121. PubMed ↩
-
Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123(18):2777-2782. PubMed ↩
-
Stahl M, Tallman MS. Differentiation syndrome in acute promyelocytic leukaemia. Br J Haematol. 2019;187(2):157-162. PubMed ↩