check point inhibitor pneumonitis
- related: Pulmonary Diseases, autoimmune encephalitis from check point inhibitor
- tags: #literature #pulmonology
This patient has developed pneumonitis from checkpoint inhibitor immunotherapy. Nivolumab and other antiprogrammed cell death receptor 1 (PD-1) or antiprogrammed cell death ligand 1 (PD-L1) monoclonal antibodies have been associated with toxicity, including pneumonitis, in an increasing number of patients treated. PDL-1 agents are used in the treatment of lung cancer and melanoma. Immune checkpoint inhibitors (ICI) work by harnessing the intrinsic immune response against tumor antigens by removing the controls on T-cell activation by antigen presenting cells. Normally, the role of the PD-1 pathway is to promote tolerance to self-antigens to prevent autoimmunity and to limit normal tissue damage. Nivolumab is a human IgG4 monoclonal antibody.
The pneumonitis following treatment with checkpoint inhibitors can range from mild to severe and fatal. ICI toxicity usually starts at a median of 2.8 months (range 9 days to 19 months) after initiating therapy. Patients present with the gradual onset of dyspnea, dry cough, and exercise desaturation usually without associated symptoms. Fever, chills, sweats, weight loss, chest pain, or hemoptysis are described but are less common. Up to one-third of patients are asymptomatic. On chest imaging (CT is the imaging procedure of choice), findings may include interstitial pneumonitis favoring nonspecific interstitial pneumonia (NSIP) (as in this case), acute interstitial pneumonitis, ground glass opacities, cryptogenic organizing pneumonia pattern with patchy consolidation/mass like formation with or without air bronchograms in a peripheral or subpleural distribution, hypersensitivity like pattern with centrilobular nodules, bronchiolitis, tree-in–bud pattern, cysts, bronchiectasis, or a mixture of nodular and other subtypes. A sarcoidal-like reaction with hilar and mediastinal adenopathy and nodular disease has also been described.
Treatment includes discontinuing the drug in mild cases and initiating corticosteroids and other immunosuppression in more severe cases. The latter should be done with caution since increasing immunosuppression could theoretically make the drug less efficacious. Other options include stopping the medicine and then reintroducing the drug or changing to an alternate agent. Other organ systems can be effected by this class of drugs including the skin (rash, dermatitis, photosensitivity, pruritus, urticaria, vitiligo, palmoplantar erythrodysesthesia, toxic epidermal necrolysis), thyroid (hypo or hyper), gastrointestinal tract (diarrhea, colitis, elevated liver function tests), kidneys (renal failure or nephritis), and musculoskeletal system.
A clinical practice guideline made suggestions for the management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy. Toxicity is graded on a scale of grades 1 to 4 based on symptoms and radiographic findings. Patients with grade 1 toxicity are usually asymptomatic with involvement of one lobe or <25% of the lung field. Management includes holding the drug and reintroducing checkpoint inhibition when there is improvement. Grade 2 patients are usually symptomatic, with more than one lobe or 25% to 50% of the parenchyma involved, and are treated with discontinuation of drug and corticosteroids (prednisone 1-2 mg/kg/d). Grade 3 disease has severe symptoms and involvement of all lobes or >50% of the parenchyma. Drug should be permanently discontinued and corticosteroids (methylprednisolone 1-2 mg/kg/d for 4-6 weeks) initiated. Stronger immunosuppression is often indicated. Grade 4 is considered life threatening with respiratory failure requiring intubation and is generally managed similarly to grade 3, but many of these patients have fatal outcomes. At each stage, if there is no improvement upon stopping the drug after 3 days (3-day intervals are recommended for follow-up and no improvement or worsening after 3 days is considered refractory disease), the management should be upgraded. In addition, grade 3 and 4 patients are usually treated empirically with antibiotics, and bronchoscopy is often performed. Additional immunosuppression may include cyclophosphamide, infliximab, IV immunoglobulin, mycophenolate mofetil, and/or tocilizumab. Rechallenge can be attempted in milder cases or those that respond well to discontinuation of therapy, but there is a 33% recurrence rate on rechallenge, and this should not be done in grade 3 or 4 cases.
The incidence of ICI toxicity is approximately 2.7% (0%-10%) when used as monotherapy and is noted to be increasing with the increased use of the drug. The incidence is 13% of all grades in lung cancer trials, with 2% grade 3 or more. The incidence may be increased with certain tumor types (melanoma may have a lower incidence), treatment with combination ICIs, and use in nontrial settings. Other postulated risk factors for development include male sex, age >60 years, and prior radiation. In some series, three-quarters of patients were diagnosed with grade 1 or grade 2 disease, 86% improved with withholding drug ± immunosuppression, and 14% progressed and died.
The diagnosis of drug toxicity is a diagnosis of exclusion and other clinical entities must be considered in the differential diagnosis. Bronchoscopy may be performed in some cases to exclude infection, particularly if recent cytotoxic chemotherapy or corticosteroid use has occurred, but it is not a requirement for diagnosis.
This patient presents with a subacute respiratory illness that is characterized by a CD8+ T-cell lymphocytic alveolitis (BAL specimen was obtained and showed increased cellularity with 71% lymphocytes, 20% neutrophils, 5% eosinophils, and 4% macrophages. On flow cytometry, the CD4+/CD8+ (cluster of differentiation) T lymphocytes ratio was 0.2.). His history, radiography, and laboratory test results are very suggestive of immune checkpoint inhibitor pneumonitis (CIP) (choice C is correct). Checkpoints, when activated, downregulate immune responses to tumor antigens, and, by inference, checkpoint inhibitors upregulate these responses. Programmed cell death-1 (PD-1) receptors are critical immune checkpoint molecules that are expressed on the cell surface of antitumor cytotoxic T lymphocytes. PD-1 can be activated by ligands expressed by certain tumor cells. Activation of PD-1 then allows the tumor cells to evade detection by cytotoxic T cells. Monoclonal antibodies that either block the PD-1 receptor (eg, pembrolizumab) or bind up PD-1 ligand will reverse the downregulation of cytotoxic T cells increasing tumoricidal activity. Cytotoxic T-lymphocyte-associated antigen 4 is another targetable immune checkpoint that functions similarly to PD-1.
Lymphangitic spread of lung cancer would be less likely to occur this soon during therapy, particularly because other areas of tumor growth are regressing and there is no prominent interlobular thickening. Unlike traditional chemotherapy, PDL-1 inhibitors enhance and do not inhibit the immune system, so cell mediated and other opportunistic infections are less common. Patients with malignancy are prone to pulmonary thromboembolism, but that is less likely with the gradual onset of symptoms and new findings in the parenchyma on chest imaging. Other considerations in the differential include ICI associated myocarditis with pulmonary edema, radiation pneumonitis if recent radiation has occurred, opportunistic infection if recent unrelated steroid use has occurred, or diffuse alveolar hemorrhage if cytotoxic chemotherapy has been used.
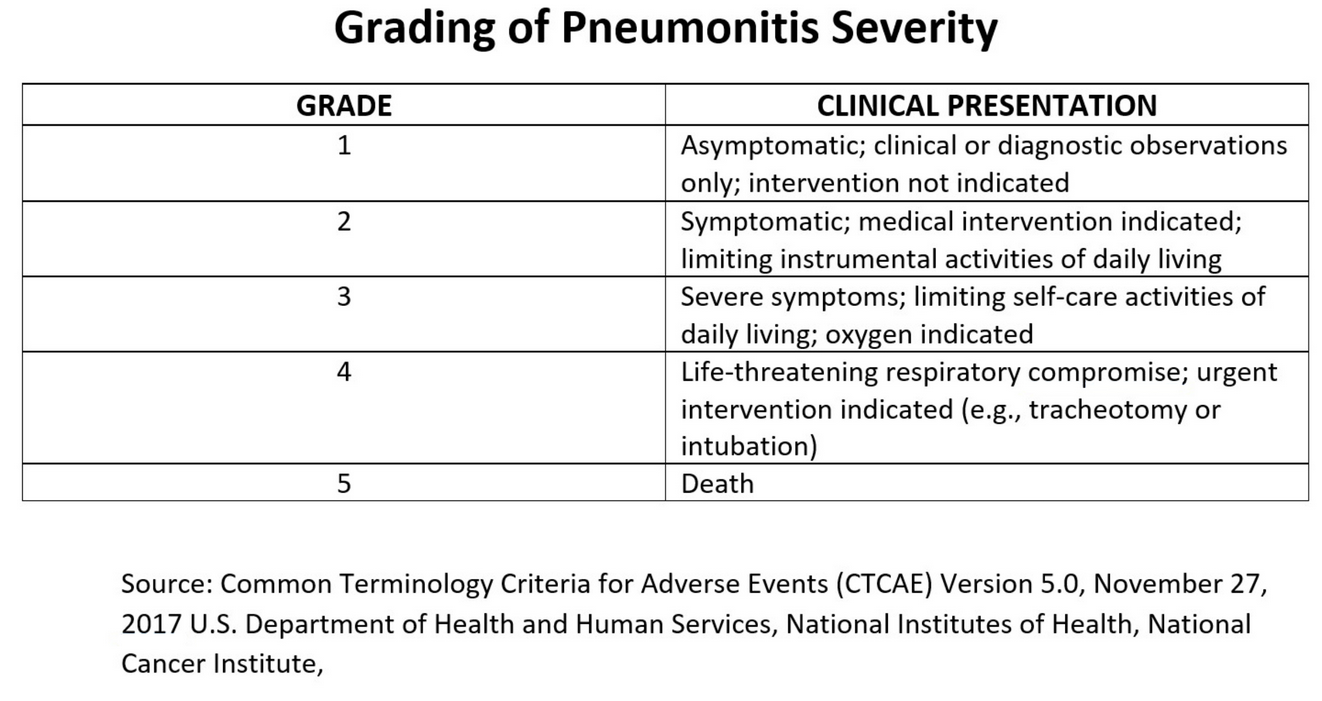
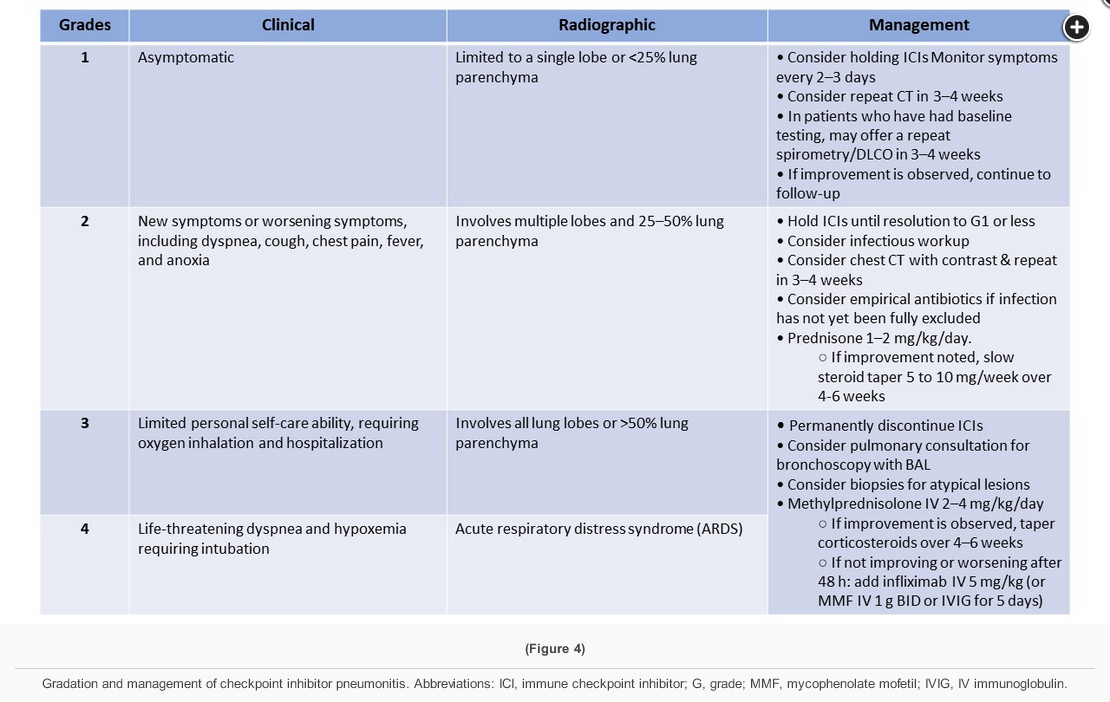
This patient has pneumonitis due to treatment with pembrolizumab, an immune checkpoint inhibitor (ICI). ICI pneumonitis is a diagnosis of exclusion and requires the rejection of other diagnostic possibilities including infection, pulmonary edema, pulmonary embolism, and exacerbation of chronic respiratory disease. ICI pneumonitis is graded from 1-5 (see Figure 9). Withholding of ICI therapy and, for grade 2 or higher toxicity, administration of glucocorticoids are the cornerstones of therapy for ICI pneumonitis. While most patients with ICI pneumonitis respond to these interventions, a minority, such as this patient, experience progression to life-threatening respiratory failure. While data are limited, addition of an additional immunosuppressive agent such as infliximab (with or without cyclophosphamide) or mycophenolate is generally indicated. This patient improved clinically after infliximab was added, and follow-up chest CT images obtained approximately 12 weeks after treatment are shown in Figures 10-12. The incidence of ICI pneumonitis is approximately 5% and is lower in patients treated with an ICI combination that includes an anti-programmed cell death protein 1 or anti-programmed cell death ligand 1 monoclonal antibody (3%) than in patients treated with an ICI combination that includes an anti-cytotoxic T-lymphocyte antigen 4 antibody (10%). The incidence of the most severe forms of ICI pneumonitis (grade ≥3) is slightly below 1%. The timing of ICI pneumonitis is variable, with a median onset of symptoms of 2.8 months after treatment. The choice to reinitiate ICI therapy after toxicity has occurred is complex and dependent on multiple factors, including the severity of the toxicity, the degree of responsiveness to immunosuppressive agents, and the availability of non-ICI treatment options.
External beam radiotherapy does not have a role in the treatment of ICI pneumonitis. ICI treatment has been associated with exacerbation of radiation pneumonitis and has induced recall radiation pneumonitis in previously irradiated lung fields up to 2 years after radiotherapy has been administered.
Therapeutic plasma exchange is a technique in which a patient’s blood is removed and then returned with their own formed blood elements but with donor plasma substituted for their own plasma, which has been removed and discarded. It is useful in the treatment of a variety of autoantibody-related conditions such as acute inflammatory demyelinating polyradiculoneuropathy (Guillain-Barré syndrome), anti-glomerular basement membrane disease (Goodpasture syndrome), myasthenic crisis, and some types of vasculitis; however, it does not have a role in the treatment of ICI pneumonitis.
Cyclosporine is a calcineurin inhibitor that reduces the production of IL-2 and several other cytokines in T-lymphocytes. While calcineurin inhibitors such as cyclosporine and tacrolimus are cornerstones of immunosuppression among solid organ transplant recipients, they do not have a role in the management of ICI pneumonitis.1234567
Links to this note
-
lymphocyte predominant BAL can suggest sarcoidosis
- low CD4:CD8 ratio could suggest check point inhibitor pneumonitis[^1]
Footnotes
-
Kalisz KR, Ramaiya NH, Laukamp KR, et al. Immune checkpoint inhibitor therapy-related pneumonitis: patterns and management. Radiographics. 2019;39(7):1923-1937. PubMed ↩
-
Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607-1616. PubMed ↩
-
Bade BC, Possick JD. Pulmonary complications of immunotherapy. Clin Chest Med. 2020;41(2):295-305. PubMed ↩
-
Beattie J, Rizvi H, Fuentes P, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer. 2021;9(2):e001884. PubMed ↩
-
Shibaki R, Akamatsu H, Fujimoto M, et al. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol. 2017;28(6):1404-1405. PubMed ↩
-
Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. PubMed ↩