chronic lung allograft dysfunction CLAD
- related: Pulmonology
- tags: #literature #pulmonary
Links to this note
Chronic lung allograft dysfunction (CLAD) after lung transplant (LT) remains a major cause of morbidity and mortality. It is a limiting factor in long-term LT survival and is the leading single cause of death after the first posttransplant year. There are two described CLAD phenotypes: bronchiolitis obliterans syndrome (BOS), which is the most common and what this patient has, and restrictive allograft syndrome (RAS), constituting 18% to 30% of CLAD. CLAD-BOS develops in up to 50% of patients by 5 years after LT; by 10 years, close to 75% of patients have CLAD. CLAD-BOS is thought to be the sine qua non of chronic rejection in LT.
CLAD typically develops more than 3 months after LT and is defined as a persistent decline (≥20%) in FEV1 when compared with the posttransplant baseline, which persists after 3 months without improvement. Clinically, patients present with nonspecific findings with dyspnea on exertion and nonproductive cough, often similar to an upper respiratory tract infection and/or a symptomatic or asymptomatic decline in spirometric results. Early on, physical examination results are typically normal, but in more advanced stages, the examination may show decreased breath sounds in BOS and crackles in RAS. CLAD-BOS manifests with pulmonary function test results showing worsening obstructive lung disease. The chest radiograph is typically unchanged compared with the posttransplant baseline. In more advanced disease, the chest radiograph can demonstrate areas of hyperinflation and possibly bronchiectasis. High-resolution CT scans may show mosaicism on expiratory views as shown in Figure 1. Bronchoscopy is then performed to exclude other causes of graft dysfunction. Direct endobronchial visualization is helpful in excluding anastomotic complications. BAL is helpful in excluding many infectious complications. Transbronchial biopsy for histopathologic examination is often attempted, if the patient is able to undergo biopsy, to exclude other causes of graft dysfunction such as acute cellular rejection, but often the histopathologic analysis of CLAD is difficult to perform. When the pathologic examination is performed in CLAD-BOS, it reveals obliterative bronchiolitis of the small airways (Figure 3, arrow pointing to small remaining bronchiole lumen), and the term "obliterative bronchiolitis" is used. There is a pathologic grading system based on the degree of airway and luminal involvement. When the bronchoscopic result for suspected CLAD-BOS is nondiagnostic, the term "bronchiolitis obliterans syndrome" is used. CLAD-RAS is characterized by upper lobe-predominant fibrotic parenchymal opacities and pleural thickening, sometimes with bronchiectasis and a restrictive defect at pulmonary function testing (total lung capacity <90% of baseline or a drop of 10% from baseline), and usually RAS has a worse prognosis. Pathologic examination may show alveolar damage; interstitial fibrosis; and interlobular septal and visceral pleural fibroelastosis, with or without obliterative bronchiolitis lesions; and bronchiectasis. Recently, donor-derived cell-free DNA detected in peripheral blood is being used as a sensitive marker for allograft dysfunction.
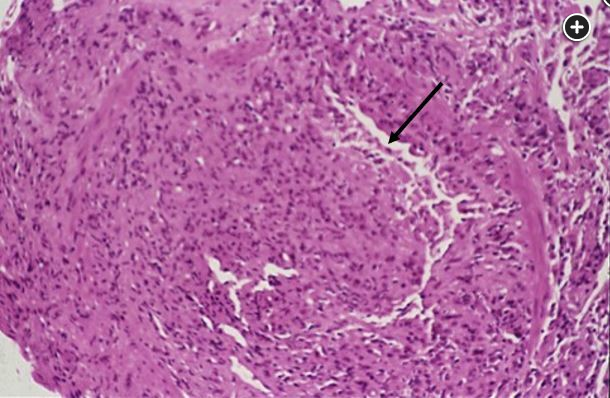
Small remaining bronchiole lumen (arrow) (×100 magnification).
CLAD staging is based on the change in FEV1 from posttransplant best. CLAD 0 is current FEV1 greater than 80% of FEV1 baseline; CLAD 1, current FEV1 greater than 65% to 80% of FEV1 baseline; CLAD 2, current FEV1 greater than 50% to 65% of FEV1 baseline; CLAD 3, current FEV1 greater than 35% to 50% of FEV1 baseline; and CLAD 4, current FEV1 35% or less of FEV1 baseline.
Risk factors for CLAD-BOS include acute cellular rejection, especially high grade and recurrent; CMV, particularly pneumonia; other viral infections; primary graft dysfunction; gastroesophageal reflux; and possibly autoimmunity. Risk factors for CLAD-RAS are less well defined, but antibody-mediated rejection or autoimmunity may be a risk factor.
Treatment for CLAD is challenging because reversal is often not possible, so stabilization is often the goal of treatment. High-dose corticosteroids are of little benefit and predispose the patient to complications, including infection. Azithromycin should be added to the regimen to slow or even slightly improve disease progression, and it also works as an immunomodulator. It is also being used as prevention at some centers. The maintenance immunosuppressive regimen should be optimized with tacrolimus and mycophenolate as the preferred combination. Other treatment options include montelukast, sirolimus or everolimus, extracorporeal photopheresis, total lymphoid irradiation, rituximab, immune globulins, and antilymphocyte or antithymocyte therapy. More experimental therapies include inhaled cyclosporine, inhaled tacrolimus, plasmapheresis, pirfenidone, alemtuzumab, and belatacept. Retransplantation may be performed for refractory CLAD, especially CLAD-BOS. Response to therapy is assessed with ongoing measurement of spirometry. The rate of progression in BOS is variable from a rapid decline, to stabilization, to a slow gradual decline, and the mortality rate is 25% to 55%. The prognosis with RAS is worse, and RAS is usually progressive.
The differential diagnosis of CLAD-BOS includes airway complications of LT, acute cellular rejection, CLAD-RAS, infection, other types of allograft rejection, and progression or recurrence of the underlying lung disease. Acute cellular rejection may be associated with a decrease in the FEV1, similar to CLAD; however, the likelihood of acute cellular rejection decreases beyond the first 6 to 12 months, although it could develop in this patient with a period of a subtherapeutic tacrolimus level. Acute cellular rejection is a histologic diagnosis with a lymphocytic vasculitis (Figure 4, arrow), not shown here. CLAD-RAS is characterized by restrictive physiology and peripheral upper lung field fibrosis, not shown or described here. CMV infection is characterized by an elevated serum CMV level at polymerase chain reaction testing and may show the classic owl's eyes on the biopsy specimen, not shown here, and with pneumonitis is associated with interstitial infiltrates. In addition, it is less likely to develop with the use of viral prophylaxis.1
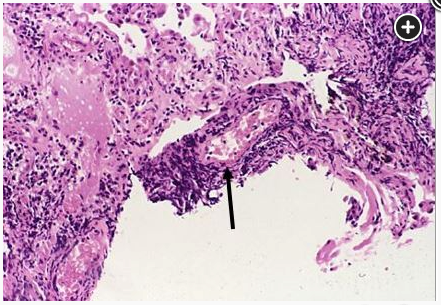
Lymphocytic vasculitis (arrow) (×100 magnification).