chronic silicone embolism syndrome
- related: Pulmonology
- tags: #literature #pulmonology
x200 and x400
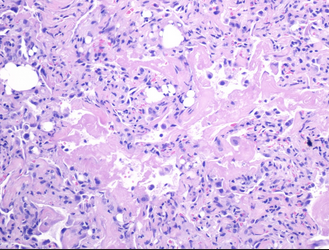
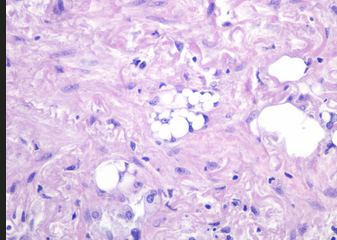
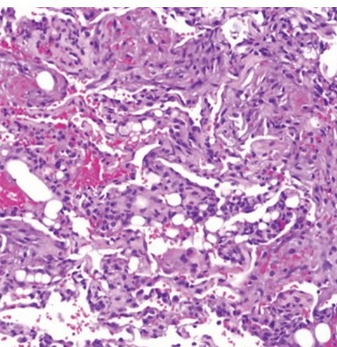
- Transbronchial lung biopsy specimen showing nonrefractile vacuole‐like structures within the alveoli (arrows). Hematoxylin and eosin stain (×50 magnification).
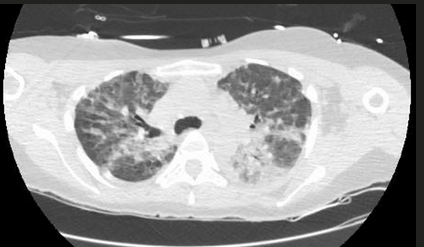
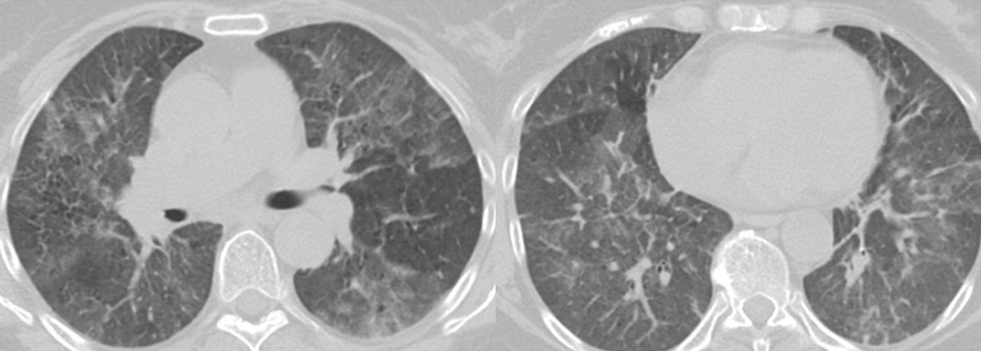
The patient had a tear in one of her breast implants and had a large chronic leak of silicone that embolized hematogenously into her lungs, causing a pneumonitis. There is disruption of the lining of the implant, as seen on the chest CT scan. There are also bilateral infiltrates seen on her chest CT scan. The lung biopsy reveals the presence of multiple clear vacuoles containing translucent, refractile, nonbirefringent microdroplets within and adjacent to alveolar septal capillaries, surrounded by histiocytes and/or multinucleated giant cells. The morphology was consistent with silicone emboli (choice D is correct).
Silicone (polydimethylsiloxane) is an inert liquid polymer used in cosmetic procedures, including breast augmentation. Case reports have been described of silicone embolization, most cases from illegal injection of silicone subcutaneously into the buttocks for augmentation. This usually causes right heart failure and ARDS. Silicone microemboli derived from breast implants (even saline breast implants with silicone casings) can potentially embolize to the lung, causing a chronic form of lung disease mimicking interstitial lung disease.
Chronic silicone embolism syndrome is characterized by chronic dyspnea, onset of lung disease years after placement of breast implants, bilateral ground-glass opacities, and pathologic evidence of silicone microdroplets in and around alveolar septal capillaries associated with histiocytes and multinucleated giant cells.
The relationship between breast implants and lung disease may not be obvious, especially in patients with saline implants (with silicone capsules), with no obvious signs of implant rupture, and a long interval between placement of implants and development of respiratory symptoms.
There is no evidence of vasculitis on the pathology specimen (choice A is incorrect). There is no history of the use of mineral oils; she is at a young age to be at risk for aspiration, and the biopsy would show vacuoles with mineral oil in a more diffuse pattern (choice B is incorrect). There is no evidence of tumor seen in the pathology specimen (choice C is incorrect).1
Liquid silicone (polydimethylsiloxane) is an inert material that is commonly used for cosmetic purposes. The use of nonmedical‐grade liquid silicone injections as an alternative to implants, although not authorized by the Food and Drug Administration, is cost-effective, and their use by unlicensed body-sculpting providers is a rising concern.
The respiratory complications associated with silicone embolism syndrome (SES) include acute pneumonitis, ARDS, alveolar hemorrhage, and silicone embolism, which can lead to cardiopulmonary failure. The clinical spectrum of SESs and the pathophysiological mechanisms behind these manifestations are poorly understood. The mechanism of silicone-induced pneumonitis is postulated to involve occlusion of the microvasculature leading to an immune-mediated inflammatory response. Embolized silicone ingested by alveolar macrophages may induce pneumonitis via local cell‐mediated inflammation and release of free radicals and proteolytic enzymes.
SES most frequently occurs within the first few days after silicone injection but can rarely happen in a latent form after a few months, with resulting fibrosis in the alveolar interstitium because of recurrent embolization. Signs and symptoms associated with SES are nonspecific and include dyspnea, hypoxia, fever, cough, and hemoptysis. Injection of large volumes of silicone into a vascular bed can cause significant embolization of the material leading to obstructive shock. Chest CT findings are nonspecific and often show diffuse peripheral and bilateral ground-glass opacities (GGOs). Diagnosis of silicone-induced pneumonitis is based on the clinical history of silicone injection, the radiological pattern of bilateral peripherally distributed GGOs, and histopathological features of nonrefractile vacuole‐like structures within the alveoli (Figure 3). There is no consensus on the treatment of SES. Other than routine supportive measures, a favorable response to corticosteroids has been noted in some case series.
Although this patient had received pembrolizumab and her clinical and radiographic findings could be consistent with immunotherapy-induced pneumonitis (IRP), the histopathological findings are inconsistent (choice A is incorrect). The development of immune checkpoint inhibitors (ICIs) has revolutionized cancer treatment. Antibodies against programmed cell death 1 (PD-1), such as pembrolizumab, increase antitumor T-cell responses by blocking the interaction between PD-1 on T cells and its ligand (PD-L1) on cancer cells. Inhibition of these proteins with monoclonal antibodies causes upregulation of the immune system allowing it to target and destroy cancer cells. However, T cells may also attack noncancerous cells resulting in immune-related adverse events, including IRP, also referred to as “checkpoint inhibitor pneumonitis” (CIP). Although uncommon, CIP is a serious and potentially fatal complication of treatment with ICIs. The incidence of CIP is approximately 5% and is lower in patients treated with an anti-PD-1 or anti-PD-L1 monoclonal antibody (3%) than in those treated with an ICI combination that includes an anti-cytotoxic T lymphocyte-associated antigen 4 antibody (10%). The incidence of the most severe forms of CIP (grade 3 or higher) is slightly less than 1% (Figure 4). CIP appears to be more frequent in patients with lung cancer, with an earlier time to onset. Tobacco smoke exposure or chronic lung diseases in patients with lung cancer could explain this difference. Lung tumor burden may also limit pulmonary endurance to external stress factors and promote pulmonary toxicity.
Presenting symptoms of CIP are nonspecific; however, because it most commonly occurs within the first 2 months of treatment, clinicians should pay particular attention to the start of treatment. The radiographic features of CIP are diverse and nonspecific. Five subtypes of radiographic patterns of CIP have been described and include cryptogenic organizing pneumonia, GGOs, nonspecific interstitial pneumonia, hypersensitivity pneumonitis, and no suggestive pattern. Acute interstitial pneumonia and ARDS patterns have also been reported. Authors of case reports have also suggested the presence of small subpleural nodules, hilar lymphadenopathy, and granulomatous changes. There are no validated recommendations for treatment of CIP. Management is currently guided by clinical experience and trial guidelines provided by the Society of Immunotherapy of Cancer and the American Society of Clinical Oncology (Figure 4).
Although this patient had received paclitaxel and her clinical and radiographic findings could be consistent with taxane-induced pneumonitis (TIP), the histopathological findings are inconsistent (choice B is incorrect). Taxanes are antimicrotubule drugs that have a broad range of antitumor activity used as standard of care for numerous malignancies. TIP is thought to be an immune-mediated delayed hypersensitivity reaction and usually occurs within days to weeks after treatment, with an incidence of 1% to 5%. Respiratory symptoms may start at the first treatment and worsen with subsequent exposure or after several cycles.
Symptoms of TIP are nonspecific and include dyspnea, dry cough, malaise, and low-grade fever. Several clinical patterns have been observed and include acute diffuse interstitial pneumonia (most severe and rapidly progresses to acute respiratory failure), subacute diffuse interstitial pneumonia (delayed onset and less severe clinical course), and pulmonary opacities with peripheral eosinophilia (marked by an indolent course and excess infiltration of eosinophils within lung interstitium and alveoli). Pulmonary fibrosis can occur as a late complication after the initial inflammatory process and may result in chronic respiratory failure. An organizing pneumonia pattern (GGOs with patchy consolidation in a subpleural or peribronchial distribution) has also been reported.
Diagnosis of TIP is usually based on a constellation of findings: a compatible clinical pattern; exposure history; and the exclusion of other causes of diffuse pulmonary opacities such as infection, radiation pneumonitis (RP), alveolar hemorrhage, reaction to other antineoplastic agents, lymphangitic carcinomatosis, and cardiogenic pulmonary edema. Treatment of TIP consists of cessation of taxane therapy. The decision to initiate corticosteroids usually depends on the severity and rapidity of worsening of the pulmonary involvement.
Although this patient had received radiation therapy for breast cancer, the radiographic and histopathologic findings are inconsistent with radiation-induced pneumonitis (choice C is incorrect). RILI can be divided into an acute phase injury (<6 months), also called “radiation pneumonitis” (RP), and a chronic phase injury (>6 months), known as “radiation fibrosis” (RF). Radiation causes several effects in the lungs, including edema, epithelial changes, disruption of the microvasculature, and atelectasis, that lead to inflammatory changes resulting in RP. Radiation induces a loss of the alveolar barrier function by destroying epithelial and endothelial cells. The inflammatory response induces a cycle of increased inflammation, vascular permeability, and cytokine release within days or weeks. Macrophage accumulation and activation contribute to the development of hypoxia, stimulating the production of reactive oxygen species and reactive nitrogen species, and proinflammatory, profibrogenic, and proangiogenic cytokines that perpetuate a nonhealing tissue response that leads to RF.
Clinical diagnosis of RP is often complicated owing to the presence of nonspecific symptoms and coexisting conditions. In RP, patients may experience an exacerbation of previous respiratory symptoms or new clinical manifestations, such as dyspnea, cough, pleuritic chest pains, and fevers. Severe signs and symptoms are less frequent but involve hypoxemia and respiratory failure. PF manifests itself as progressive respiratory insufficiency or worsening severity of previous symptoms, particularly dyspnea.
Although RILI with symptoms occurs in a relatively small number of patients, a significantly higher percentage of patients (43%) will eventually have radiologic changes. The late changes of RF usually begin 6 to 9 months after the course of radiotherapy and become stable after 2 years. Chest CT findings in RP typically align closely with the irradiated area. Perivascular GGOs are an early radiation-induced abnormality, followed by an organizing phase that is typically associated with patchy areas of consolidation that coalesce to form a relatively sharp edge that conforms to the radiation therapy portals rather than anatomic structures (Figure 5). A “straight line” effect, which does not conform to anatomical units but rather to the confines of the radiation port, is virtually diagnostic of RILI. The airspace opacities associated with the organizing phase may resolve with minimal scarring or may evolve into a fibrotic phase, characterized on CT scans by linear reticular opacities or an area of dense consolidation and volume loss (Figure 6).
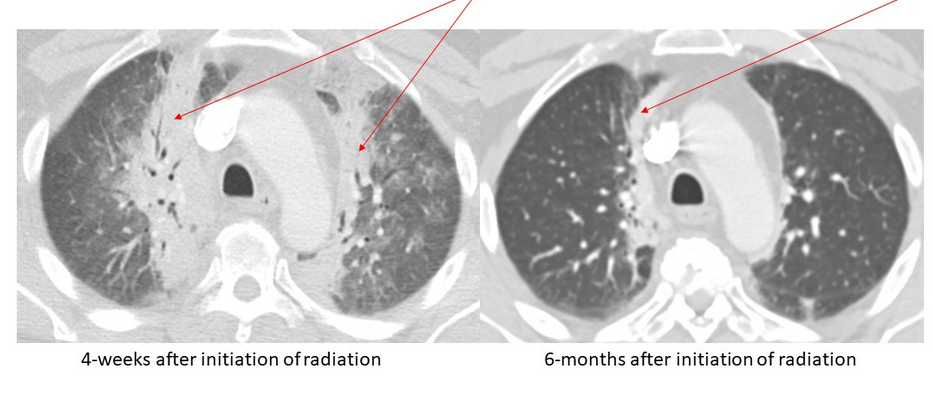
- Axial chest CT scans (lung windows) obtained in a 50-year-old man with T4/5 pathologic fractures (arrows) secondary to metastatic prostate cancer requiring radiation therapy.
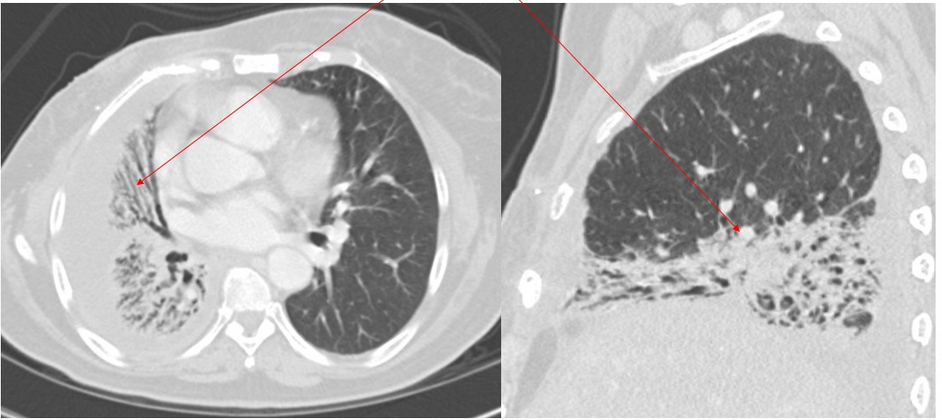
- Axial and sagittal chest CT scans (lung windows) obtained in a 60-year-old woman with right-sided breast cancer with radiation fibrosis (arrows).
The diagnosis of RILI is commonly based on a combination of typical symptoms; timing, dose, and location of radiation therapy; compatible imaging findings; and exclusion of other causes, most notably infections. Investigators in few controlled studies have evaluated the efficacy of treatment for RILI; therefore, optimal treatment is unknown. Corticosteroids can be considered for patients with symptoms with subacute onset of RP. Patients with RF due to previous irradiation are unlikely to benefit from such therapy.