decrease flow rate in status asthmaticus over increasing PEEP
- related: ICU intensive care unit, Asthma reactive airway disease
- tags: #literature #icu
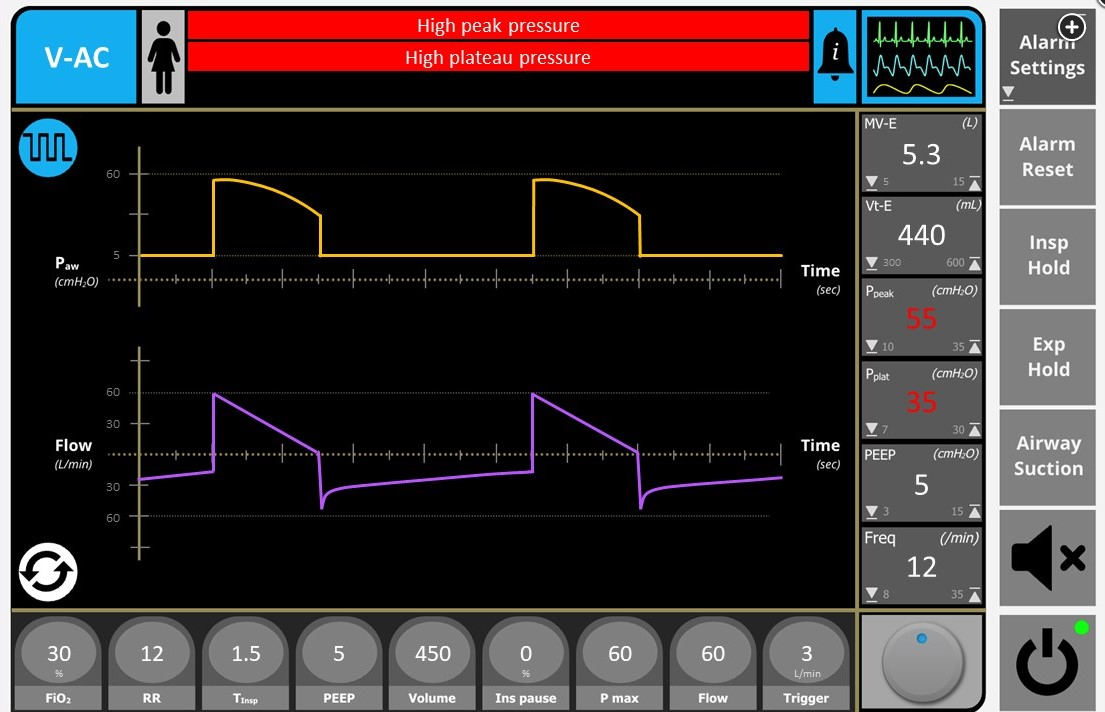
Figure 1 demonstrates an elevated peak inspiratory pressure, a significant plateau pressure gradient, and continuing flow at end expiration. These findings in this clinical scenario are strongly suggestive of a diagnosis of status asthmaticus. General principles of mechanical ventilation in status asthmaticus include controlled hypoventilation with a low tidal volume, respiratory rate, and long expiratory time to reduce dynamic hyperinflation and the resulting risk of barotrauma. Of the choices available, decreasing the respiratory rate is the most reasonable first option to consider in this setting.
The equation of motion offers a helpful conceptual framework to understand the physiologic rationale for mechanical ventilation management in status asthmaticus. If the force used to drive ventilation is equal to the force applied by the respiratory system in the opposite direction, then the total pressure required to generate a certain level of ventilation is the sum of the pressure delivered by the ventilator (Pvent) and the pressure generated by the patient’s respiratory muscles (Pmus, presumably 0 here, in this setting of pharmacologic neuromuscular blockade).
Pmus + Pvent = resistive load + elastic load
Pmus + Pvent = (resistance × flow) + (elastance × volume) + pressure at end expiration + inertance
Respiratory system pressure is generated by the sum of its resistive (dynamic) and elastic (static) loads, the pressure at end expiration, and inertance (the inertial components required to move gas). In this case, the resistive load is generated by the product of the flow delivery of the mechanical breath (set at 60 L/min) and the resistance generated by the ventilator circuit, endotracheal tube, and patient airways. In life-threatening asthma, increased airways resistance is the major contributing factor to this resistive load.
Elastic load is defined as the product of respiratory system elastance (change in pressure divided by change in volume) and change in volume (tidal volume, here set at 450 mL). The elevated airways resistance in status asthmaticus leads to expiratory flow limitation and dynamic hyperinflation, which causes increased respiratory system elastance with elevated plateau pressure (measured during a pause at end inspiration) and pressure at end expiration (which is elevated above the set PEEP owing to intrinsic PEEP [iPEEP]).
Importantly, iPEEP measurement often underestimates the degree of regional dynamic hyperinflation that is present. Mucus plugs and dynamic airway compression at end expiration can lead to heterogeneity of alveolar ventilation and place patients at risk for barotrauma (pneumothorax, pneumomediastinum). Determinants of dynamic hyperinflation include degree of airflow obstruction, tidal volume, and expiratory time. Decreasing respiratory rate and tidal volume prolongs expiratory time and reduces the amount of gas that must be exhaled, and permissive hypercapnia can be employed when needed to reduce the risk of barotrauma. While increasing the extrinsic PEEP setting on the ventilator to approximate measured iPEEP can reduce the risk of trigger dyssynchrony in a spontaneously breathing patient, in this pharmacologically paralyzed patient, this change offers little benefit at this time. Reducing the flow rate in a volume-targeted ventilator mode will lengthen the inspiratory and shorten the expiratory times, potentially worsening dynamic hyperinflation. Elevated peak inspiratory pressures owing to high resistive loads are common early in the mechanical ventilation course of status asthmaticus and are generally associated with lower risk of barotrauma than elevated plateau pressures from hyperinflation.1234
A 28-year-old patient presents to the emergency department in respiratory distress several days after developing an upper respiratory infection. The patient continues to deteriorate despite initial treatment and requires rapid sequence intubation using ketamine and rocuronium.
On ICU arrival, the patient is sedated with propofol and remains pharmacologically paralyzed. Temperature is 37.8 °C, heart rate is 123/min, BP is 134/92 mm Hg, and SpO2 is 96% with bag valve mask ventilation support. The patient is placed on volume-targeted assist-control ventilation using a decelerating waveform and flow rate of 60 L/min with rate of 14/min, tidal volume of 450 mL, FIO2 of 0.5, and PEEP of 5 cm H2O. A high-pressure alarm is immediately triggered with early breath termination and minimal tidal volume delivery. After adjustment of the high-pressure alarm, peak inspiratory and plateau pressures are 55 and 35 cm H2O, respectively. Associated ventilator waveforms are displayed in Figure 1. Breath sounds are reduced bilaterally, with a prolonged expiratory phase. Admission portable chest radiograph is shown in Figure 2, and bilateral sliding lung is readily identified throughout the thorax using bedside ultrasonography. Arterial blood gas analysis performed after intubation shows pH of 7.32, PaCO2 of 50 mm Hg, and PaO2 of 113 mm Hg.
Which of the following ventilator setting changes is most appropriate at this time?
This clinical presentation is most consistent with dynamic hyperinflation in the setting of status asthmaticus, with increased peak-to-plateau pressure gradient due to increased airway resistance and significant intrinsic PEEP (iPEEP) based on the elevated end-expiratory pressure above the set extrinsic PEEP. General principles of mechanical ventilation in the patient with status asthmaticus to avoid complications of dynamic hyperinflation include controlled hypoventilation with low tidal volume, low respiratory rate, and long expiratory time. Of the choices available, decreasing the respiratory rate is the most reasonable first option to consider in this setting.
Determinants of dynamic hyperinflation include degree of airflow obstruction, tidal volume, and expiratory time. Decreasing respiratory rate and tidal volume prolongs expiratory time and reduces the amount of gas that must be exhaled. In cases of severe airflow obstruction, a permissive hypercapnia strategy may be necessary to avoid the complications of barotrauma. Changing the inspiratory trigger threshold will have no impact in a mechanically ventilated patient receiving neuromuscular blockade, although in a spontaneously breathing patient, reducing the inspiratory trigger threshold may be helpful in the setting of trigger dyssynchrony and elevated levels of iPEEP.
Similarly, while increasing the set PEEP on the ventilator to approximate measured iPEEP can also reduce the risk of trigger dyssynchrony in a spontaneously breathing patient with dynamic hyperinflation, this change offers little benefit in this pharmacologically paralyzed patient. Reducing the flow rate in volume-targeted ventilator mode will lengthen inspiratory and shorten expiratory times, potentially worsening dynamic hyperinflation.
You are called to evaluate a 22-year-old patient because of a high-pressure ventilatory alarm. The patient was admitted earlier the same day for acute hypoxemic and hypercapnic respiratory failure due to status asthmaticus and was intubated. The patient is sedated with propofol and is receiving therapeutic neuromuscular blockade. Temperature is 37.8 °C, heart rate is 132/min, and BP is 105/74 mm Hg on volume-targeted assist-control ventilation using a decelerating waveform and flow rate of 60 L/min with respiratory rate of 12/min, tidal volume of 450 mL, FIO2 of 0.4, and PEEP of 5 cm H2O. Recent arterial blood gas analysis shows a pH of 7.36, PaCO2 of 45 mm Hg, and PaO2 of 89 mm Hg. Peak inspiratory and plateau pressures are 55 and 35 cm H2O, and pressure at end-expiration is 10 cm H2O. The endotracheal tube is patent, and breath sounds are symmetric but reduced bilaterally. Bilateral sliding lung is readily identified using bedside ultrasonography. Repeat chest radiograph demonstrates hyperinflation only.
Which of the following ventilator setting changes is most appropriate at this time?
Links to this note
Footnotes
-
Garner O, Ramey JS, Hanania NA. Management of life-threatening asthma: severe asthma series. Chest. 2022;162(4):747-756. PubMed ↩
-
Hess DR. Respiratory mechanics in mechanically ventilated patients. Respir Care. 2014;59(11):1773-1794. PubMed ↩
-
Mannam P, Siegel MD. Analytic review: management of life-threatening asthma in adults. J Intensive Care Med. 2010;25(1):3-15. PubMed ↩