EVALI ecigarette or vaping associated lung injury
- related: Pulmonology
- tags: #literature #pulmonology
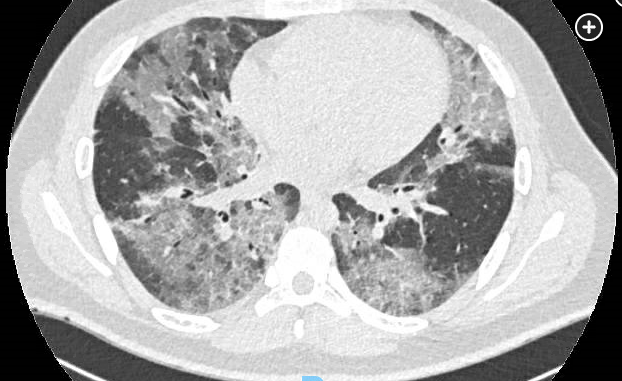
The term vaping has been coined to describe inhalation of various aerosols resulting from heating either a liquid or resin composed of nicotine or other compounds such as a variety of flavorings, various additive agents such as glycerol or propylene glycol, and cannabinoids. A small, portable cartridge or similar device outfitted with a battery (e-cigarettes), or vape pens, are used to deliver the aerosol. Beginning in 2019, a cluster of cases comprising individuals who were vaping was identified. These patients experienced acute to subacute lung injury characterized by shortness of breath, cough, chest pain (often pleuritic in nature), subjective fever and chills, and occasionally hemoptysis. This condition was termed e-cigarette or vaping product use-associated lung injury (EVALI). GI symptoms were common and included nausea or emesis as well as diarrhea and abdominal pain. More than 3,000 cases of EVALI have been reported to the Centers for Disease Control and Prevention, with as many as 75 deaths.
The precise pathogenesis of EVALI is unclear but appears to be a form of acute lung injury with pathologic findings of acute fibrinous pneumonitis, diffuse alveolar damage, or organizing pneumonia that is typically accompanied by bronchiolitis. No evidence of an infectious etiology has been noted, and EVALI may actually represent an array of various insults to the pulmonary parenchyma, as opposed to a discrete entity. Several descriptions have arisen from pathologic specimens in these individuals, such as acute eosinophilic pneumonia, diffuse alveolar hemorrhage, lipoid pneumonia, and respiratory bronchiolitis interstitial lung disease, suggesting that the pathogenesis is multifactorial. The key risk factor for EVALI has been active vaping by the patient.
While lung injury associated with vaping has been described for many years at low rates, in fall 2019, an outbreak of severe lung injury in previously healthy patients who were using e-cigarettes created substantial new interest. After rapid investigation, it was determined that the bulk of the 2019 outbreak was caused by vitamin E acetate used as an adulterant in tetrahydrocannabinol (THC) vaping liquids. (Artisanal/informal manufacturers used the vitamin E acetate to covertly dilute the THC in vaping liquid to maximize their profits.) The initial evaluation of the EVALI outbreak included identification of lipid-laden macrophages on BAL as well as vitamin E acetate in lavageate and in the implicated THC vaping liquid.
While vitamin E acetate was etiologic in the 2019 outbreak, it is much less common now and, in any case, is not required to make the diagnosis of EVALI (choice A is incorrect). The acute outbreak decreased substantially after identification of the vitamin E acetate, the apparent removal of that adulterant, and, perhaps, decreased use of adulterated THC vaping liquid. Nevertheless, EVALI continues to the present day, albeit with less frequency than during the 2019 outbreak.
Although various waxes are used in certain e-cigarette devices, liquefied paraffin has not been among the materials associated with EVALI. Hydrated magnesium silicate (talc) has been associated with talc pneumoconiosis and is one of the rarer forms of silicate-induced lung disease. It has been described in workers exposed to talc during its production or its industrial use and reported in IV drug users but has not been noted in cases of EVALI. Volatile organic compounds, such as benzene, ethylene glycol, or formaldehyde, may include a variety of products that may result in eye, nose, and throat irritation; shortness of breath; headaches; fatigue; nausea; dizziness; and skin problems. Higher concentrations may cause irritation of the lungs, as well as damage to the liver, kidney, or CNS. However, volatile organic compound inhalation has not been noted as having a strong association in those individuals developing EVALI.1
Although lipid-laden macrophages are common in patients with EVALI, they are not required to make the diagnosis. In fact, BAL is not routinely recommended in the evaluation of patients with possible EVALI (choice B is incorrect).
No randomized controlled trials guide treatment of EVALI. Based on case series, common therapy for EVALI, other than supportive care and cessation of vaping, includes steroids, which anecdotally yielded excellent response among patients with EVALI related to vitamin E acetate. Lower moderate-dose steroids for short courses were used during the 2019 outbreak. However, whether steroids have therapeutic efficacy in EVALI unrelated to vitamin E acetate is unknown. Most authors strongly emphasize the importance of cessation from vaping and/or e-cigarette use.2345
Links to this note
Footnotes
-
Aberegg SK, Cirulis MM, Maddock SD, et al. Clinical, bronchoscopic, and imaging findings of e-cigarette, or vaping, product use-associated lung injury among patients treated at an academic medical center. JAMA Netw Open. 2020;3(11):e2019176. PubMed ↩
-
Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK, Lanspa MJ. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019;394(10214):2073-2083. PubMed ↩
-
Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. PubMed ↩
-
Centers for Disease Control and Prevention. For State, Local, Territorial, and Tribal Health Departments. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html. Accessed April 8, 2022. ↩