invasive pulmonary aspergillosis
- related: pulmonary aspergillosis
- tags: #literature #pulmonology #id
Invasive pulmonary aspergillosis:
- most often occurs in immunosuppressed patients who are neutropenic or are HSC transplant recipients
- starts in respiratory tract and then enters the circulatory system (angioinvasion). This is followed by the formation of a fungus-septic emboli complex with subsequent hematogenous dissemination.
- Sx: most commonly pulmonary aspergillosis (60%), but sinusitis, brain abscess, endocarditis, disseminated infection, and osteomyelitis also may occur.
- fever unresponsive to antibiotics
- angioinvasive: hemoptysis
- Dx:
- blood cultures generally negative
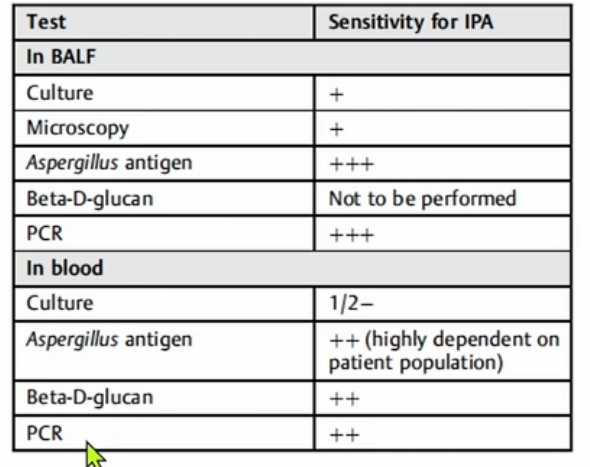
- serum galactomannan test testing is recommended
- If the serum galactomannan result is negative, but the patient has strong risk factors, bronchoalveolar lavage with galactomannan and Aspergillus polymerase chain reaction (PCR) are recommended, followed by tissue biopsy, if necessary, to establish a definitive diagnosis.
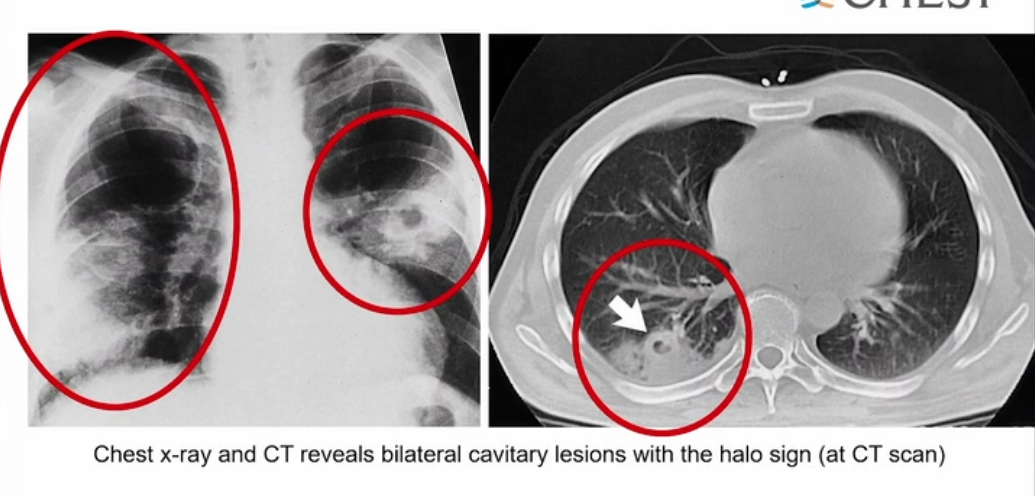
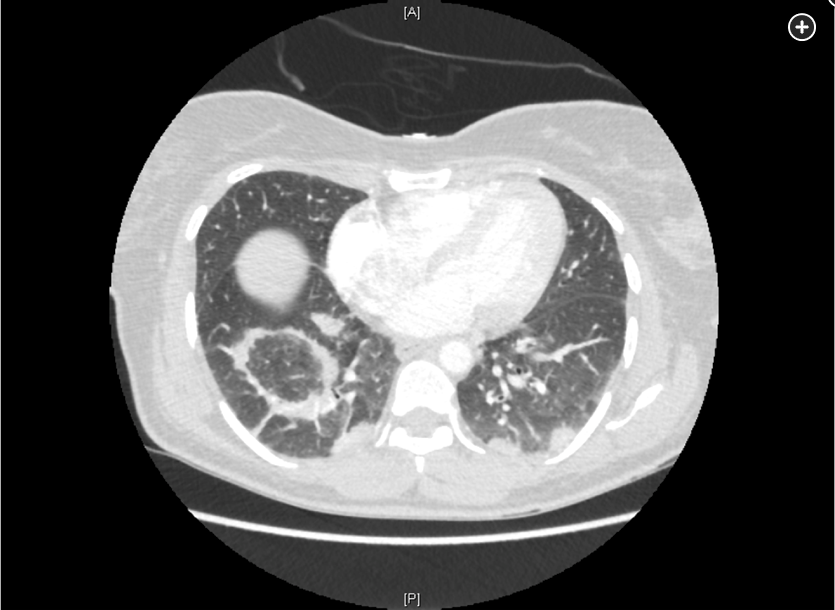
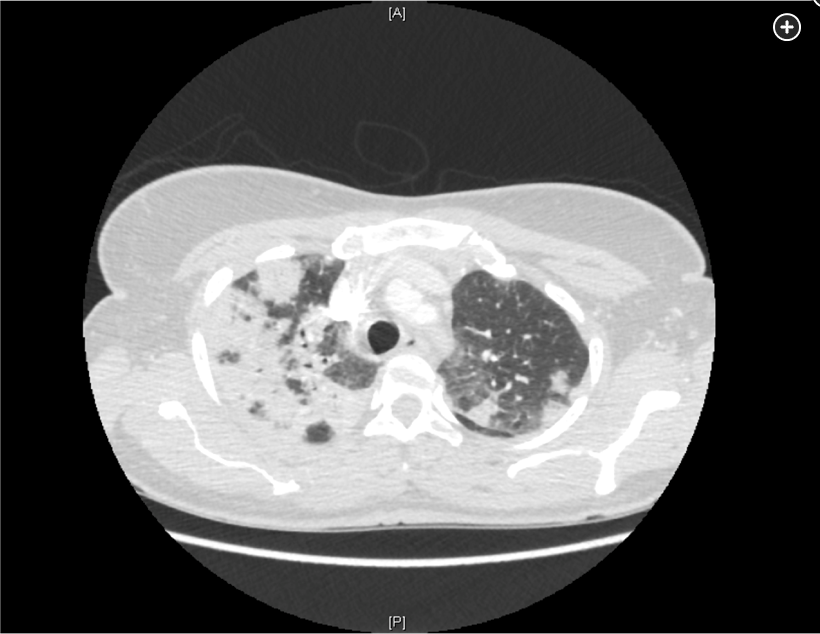
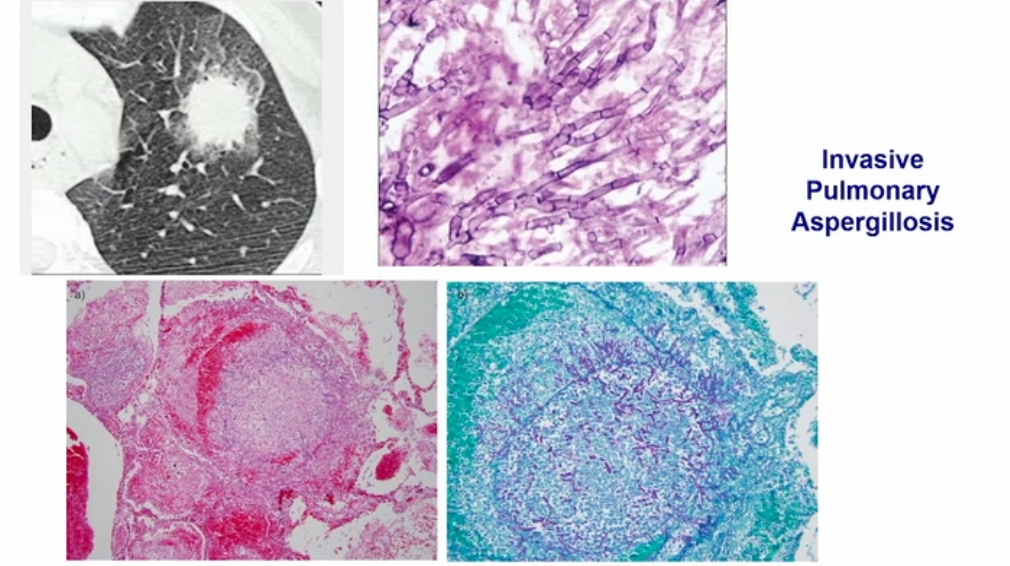
- Early CT of the chest in patients with suspected invasive pulmonary aspergillosis—with typical findings suggestive of septic emboli (nodules, often with a “halo sign”), thromboembolic pulmonary infarction (wedge-shaped peripheral densities), or necrosis with cavitation and a crescent sign—and the serum galactomannan assay can be useful in establishing a presumptive diagnosis of invasive infection, especially in neutropenic patients and hematopoietic stem cell transplant recipients, in whom the assay has a sensitivity of approximately 80%.
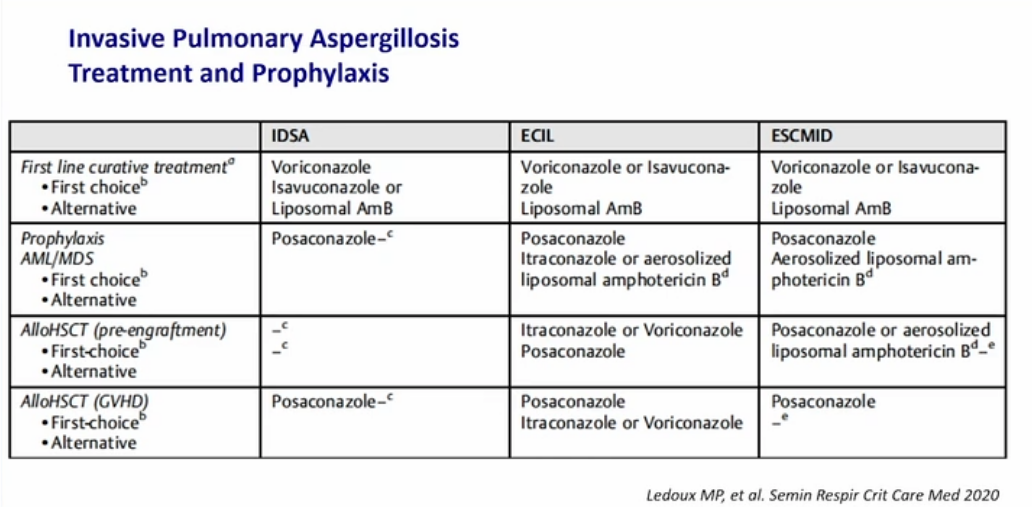
- First-line treatment of invasive aspergillosis is with a triazole (voriconazole, posaconazole, or isavuconazole); amphotericin B deoxycholate or liposomal amphotericin B can be used for amphotericin-sensitive Aspergillus species if triazoles a re not available. When possible, reversal of immunosuppression improves treatment response.
- Several antifungals have shown effectiveness in treating IPA. Early on, voriconazole demonstrated superiority to amphotericin, and isavuconazole has subsequently demonstrated equivalence to voriconazole. Amphotericin is often an effective drug for other fungal infections that are either very serious or may involve the CNS, but voriconazole and isavuconazole both cross the blood-brain barrier
The most rapidly progressive and lethal consequence of exposure to Aspergillus spores is invasive aspergillosis, which occurs in patients who are immunosuppressed, with the lung the most commonly recognized site of infection, although dissemination via circulation can occur. Typical settings include patients who are neutropenic undergoing bone marrow transplant, solid organ transplant recipients, and patients undergoing CAR-T cell therapy, especially if it has been complicated by cytokine release syndrome, which requires treatment with tocilizumab and corticosteroids (CAR-T and cytokine release syndrome), as in the patient presented. Invasive aspergillosis has also been described in patients with advanced cirrhosis and after prolonged courses of respiratory failure managed with mechanical ventilation in the ICU, including COVID-19 pneumonitis.
Diagnosis of invasive aspergillosis would ideally be based on tissue biopsy showing invasion with organisms showing the typical acutely branching hyphal morphology consistent with Aspergillus species and positive culture (see Figure 4). Biopsy is often not possible in patients who are critically ill, and alternative diagnostic methods may be employed. Testing for fungal antigens such as galactomannan or β-D-glucan can be performed on serum and/or BAL fluid, and a serum polymerase chain reaction test is available from reference laboratories. Serum tests for galactomannan and β-D-glucan were strongly positive in this patient. Radiologic findings can also strongly suggest a diagnosis of invasive fungal disease. Often, there are dense nodular infiltrates (as seen on the CT scan performed on this patient), and there may be halo or reverse halo signs. The halo sign consists of ground glass density surrounding a dense nodule, as shown in Figure 5. It is thought to correlate with areas of hemorrhage surrounding denser areas of active infection, and pathologically invasive aspergillosis is characterized by areas of hemorrhage and infarction. The reverse halo sign, which was present on the CT scan of this patient (see Figure 6, arrow), is described as a rim of high attenuation surrounding an area that likely represents infarction.
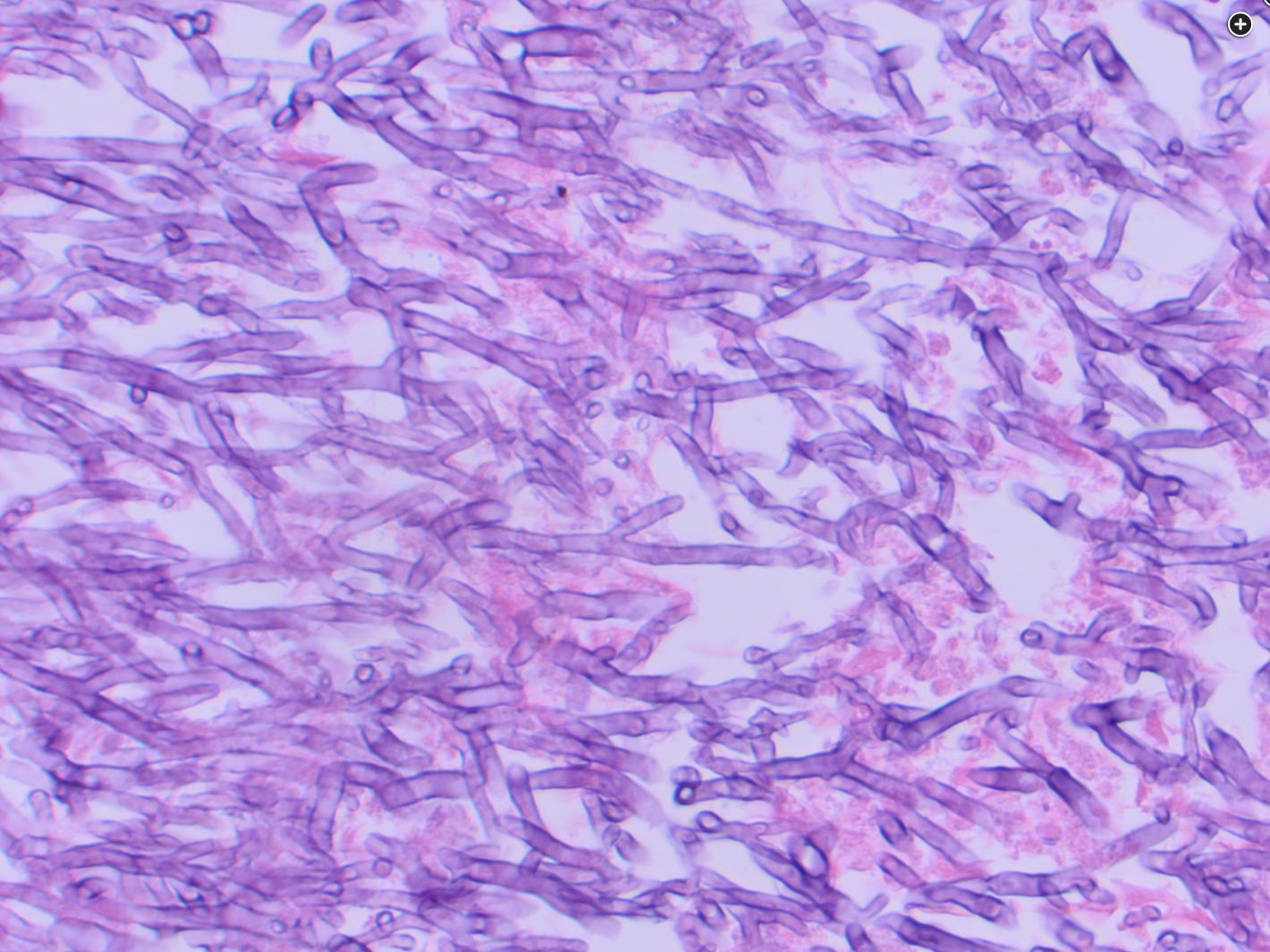
- septated hyphae differentiates from mucormycosis
- use septation and branching angle to differentiate between mucor and aspergillosis
- voriconazole fist line treatment
Covid associated pulmonary aspergillosis
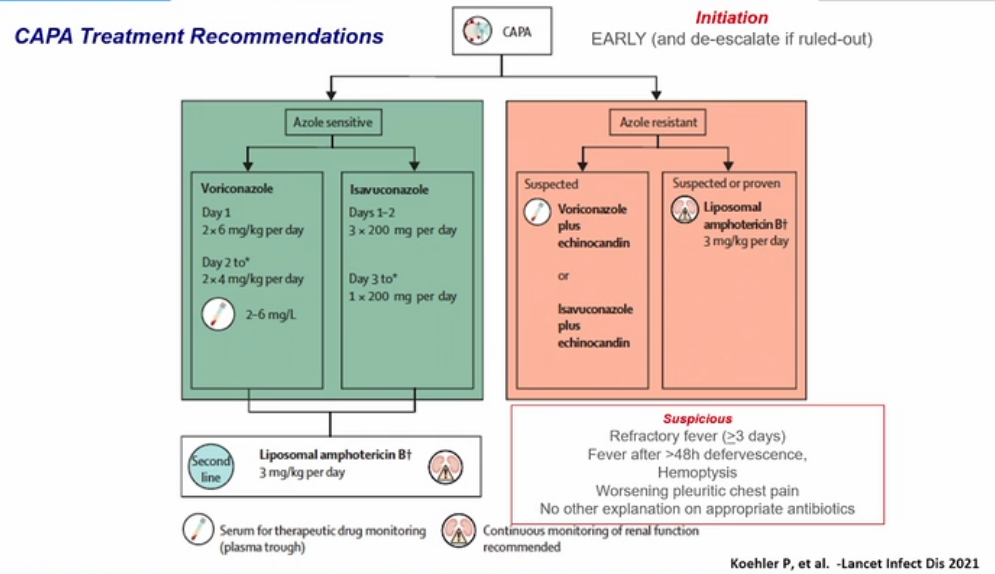
- if resistant use echinocandin and amphotericin B
Treatment of invasive aspergillosis centers on use of newer generation azoles. While amphotericin B was a mainstay of treatment in the past, a trial comparing it with voriconazole found improved outcome with the azole. A recently published trial showed posaconazole to be equivalent to voriconazole in terms of survival and without as severe a side effect profile. Therapeutic drug monitoring is recommended for posaconazole. Amphotericin B would be the antifungal treatment of choice if this patient suffered from mucormycosis, another cause of lung infection in patients such as this, but mucor would not cause a positive galactomannan test.
Itraconazole has a role in the treatment of allergic bronchopulmonary aspergillosis but is not recommended in the treatment of invasive aspergillosis.
While there has been interest in use of echinocandins such as anidulafungin as part of combination therapy for invasive aspergillosis, it is not currently recommended as monotherapy.123456
Symptoms of IPA are nonspecific and usually mimic bronchopneumonia: fever unresponsive to antibiotics, cough, sputum production, and dyspnea. Patients may also present with pleuritic chest pain (due to vascular invasion leading to thromboses that cause small pulmonary infarcts) and hemoptysis, which is usually mild. IPA is one of the most common causes of hemoptysis in neutropenic patients. Chest x-ray may not detect early-stage disease (ie, small nodules). Chest computed tomography (CT) scan shows single or multiple nodules with or without cavitations and “halo sign” (nodules surrounded by ground-glass infiltrates). Diagnosis is suggested by culture and histology.
Enzyme-linked immunosorbent assay on serum or bronchoalveolar lavage fluid can be used to detect galactomannan antigen (present in Aspergillus cell walls), especially in patients with hematologic malignancies. Voriconazole has become the gold standard as primary therapy for invasive aspergillosis. Voriconazole was more effective than amphotericin B as initial therapy for IPA and was associated with significantly improved survival (71% vs 58%, respectively) in a randomized trial (Choice A).
This patient likely has invasive aspergillosis complicating her COVID-19 pneumonia, as strongly suggested by the numerous hyphae branching at acute angles on the silver stain. It is important to distinguish Aspergillus species from Mucormycosis species which have a similar appearance on Gömöri methanamine-silver staining, but the latter branch at 90-degree angles and lack septations. The acute branching angles of the hyphae here and the septations seen strongly favor a diagnosis of aspergillosis, although definitive diagnosis would depend ultimately on culture. Given the strong likelihood of aspergillosis, a newer-generation azole, such as isavuconazonium, posaconazole, or voriconazole, would be recommended as primary therapy.
Invasive infection typically occurs in patients with significant immunosuppression and is the most rapid and lethal of the forms of _Aspergillus-_related disease. Most commonly, invasive infection begins with proliferation of fungal organisms in lung tissue, often with local necrosis followed by hematogenous spread of the organism to distant tissue sites. Aspergillus fumigatus is the most common cause of lung infection while wounds, especially in burn victims, more typically involve Aspergillus niger and Aspergillus flavus.
Clinicians have long been familiar with risk factors for pulmonary invasive aspergillosis, including prolonged neutropenia, bone marrow transplantation, and solid organ transplantation; however, there has been increasing recognition of this typically opportunistic infection in patients with prolonged ICU stays, including protracted periods of mechanical ventilation for viral pneumonia including infection by influenza and SARS-CoV-2, as in the patient presented here. This latter phenomenon has been termed covid-associated pulmonary aspergillosis (CAPA). In the context of the ICU, invasive pulmonary aspergillosis does not arise with radiologic evolution of nodular and necrotic change superimposed upon more normal lung fields but rather is often obscured by the cause of respiratory failure prompting initial ICU admission. Hence, an appropriate index of suspicion of Aspergillus infection must be present when evaluating patients who are critically ill with evidence of late (>10-14 days) complicating ICU infection. Diagnosis requires identification of the organism in tissue and/or body fluids and can also be supported by the serologic identification of components of the fungal cell wall, including galactomannan and 1,3-ß-D-glucan.
Since mortality from this superinfection remains high, early treatment is important. Several decades ago, the mainstay of treatment of invasive aspergillosis was administration of amphotericin. This practice changed after a randomized trial demonstrated improved survival with posaconazole as compared with amphotericin with decreased side effects. Since azoles undergo extensive hepatic metabolism via the CYP450 system, drug-drug interactions are common, and monitoring drug levels is recommended during use of voriconazole or posaconazole. Isavuconazole has fewer significant drug interactions, and in most patients, drug levels do not need to be monitored; isavuconazole has also been shown to have a lower side effect incidence than other azoles in some studies. Itraconazole, while used in other forms of aspergillosis including ABPA and cavitary disease, is not recommended for the treatment of invasive aspergillosis. Trimethoprim-sulfamethoxazole is useful in treating a number of infections, including infection from various species of Nocardia. Nocardiosis does occur in immunocompromised hosts but is not typically seen in this clinical context, and the causative organism is a weakly staining acid-fast bacillus with branching, not the silver-stain positive fungus shown here.789101112
Links to this note
Footnotes
-
Hage CA, Carmona EM, Evans SE, Limper AH, Ruminjo J, Thomson CC. Summary for clinicians: microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. Ann Am Thorac Soc. 2019;16(12):1473-1477. PubMed ↩
-
Maertens JA, Rahav G, Lee DG, et al; study investigators. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021;397(10273):499-509. PubMed ↩
-
Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. PubMed ↩
-
Thompson GR 3rd, Young JH. Aspergillus infections. N Engl J Med. 2021;385(16):1496-1509. PubMed ↩
-
Zou M, Tang L, Zhao S, et al. Correction: systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS One. 2017;12(12):e0190459. PubMed ↩
-
Gangneux JP, Dannaoui E, Fekkar A, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 2022;10(2):180-190. PubMed ↩
-
Herbrecht R, Denning DW, Patterson TF, et al; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415. PubMed ↩
-
Huang SF, Ying-Jung Wu A, Shin-Jung Lee S, et al; GREAT working group. COVID-19 associated mold infections: review of COVID-19 associated pulmonary aspergillosis and mucormycosis. J Microbiol Immunol Infect. 2023;56(3):442-454. PubMed ↩
-
Lamoth F, Calandra T. Pulmonary aspergillosis: diagnosis and treatment. Eur Respir Rev. 2022;31(166):220114. PubMed ↩
-
Thompson GR 3rd, Young JH. Aspergillus Infections. N Engl J Med. 2021;385(16):1496-1509. PubMed ↩
-
Townsend L, Martin-Loeches I. Invasive Aspergillosis in the intensive care unit. Diagnostics (Basel). 2022;12(11):2712. PubMed ↩