mesothelioma
- related: Pulmonology
- tags: #literature #pulmonology
Cause
Malignant mesothelioma commonly takes years to develop—in some series up to 40 years—and has been associated with individuals with exposure to asbestiform fibers, either from occupational inhalation or from secondhand inhalation of fibers from exposure to clothing or other items contaminated with asbestos by family members of workers in asbestos-related trades.
Smoking does NOT increase the risk of mesothelioma in asbestos workers. This is in contrast to the synergistic effect of smoking and asbestos exposure on developing primary bronchogenic lung carcinoma.
pulmonary diseases that are less common among smokers than nonsmokers
Patient and Symptoms
Asbestos-induced lung carcinomas have an extended latency period of 20 to 40 years following initial exposure. Individuals with asbestos exposure have been noted to have rates of lung cancer greater than the general population, and in those who also smoke cigarettes, even higher rates of bronchogenic carcinoma have been recorded. The incidence of malignant pleural mesothelioma, another asbestos-related disease affecting the mesothelial tissue surrounding the lung, began to escalate beginning in the 1940s, with a 5% to 10% annual increase until the past several years when incidence rates leveled off. The most important prognostic factor in each of these malignancies is disease stage. With early detection, long-term survival may not only be achievable but also >80% for early-stage non–small cell lung cancers. Selected patients with malignant pleural mesothelioma may also experience improved outcomes with aggressive therapies using combinations of chemotherapy, surgery, and radiation—if diagnosed at an earlier stage. Therefore, identifying effective screening tools is critical to curbing mortality from primary lung or pleural-based carcinomas.
Multiple studies have looked at the efficacy of LDCT as a tool for lung cancer screening in general. A number of studies in the past decade have shown that LDCT is at least four times as sensitive as chest radiographs in detecting small, potentially resectable lung cancers, albeit mortality benefits remain less clear and early detection must be balanced against identification of lesions that require investigation and are ultimately found to be benign. Large-scale studies such as the National Lung Screening Trial show that chest LDCT may identify lung cancers at an early stage in high-risk populations, such as heavy smokers, with resulting reductions in mortality rates. Other high-risk populations, such as individuals exposed to asbestos, have been studied in more detail recently, but there are no clinical guidelines tailored specifically to frequency of screening in high-risk populations. Several other studies have demonstrated that LDCT screening in asbestos-exposed workers is effective in detecting asymptomatic lung cancers with an efficacy at least equal to the prevalence noted in heavy smokers but not greater (choices A and C are incorrect). Clinical trials that have included enrollment of more than 1,000 asbestos-exposed workers and former workers have not identified statistically or clinically significant data supporting routine LDCT screening specifically for malignant pleural mesothelioma (choice B is incorrect).
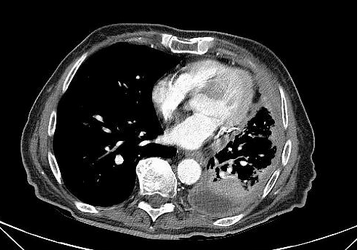
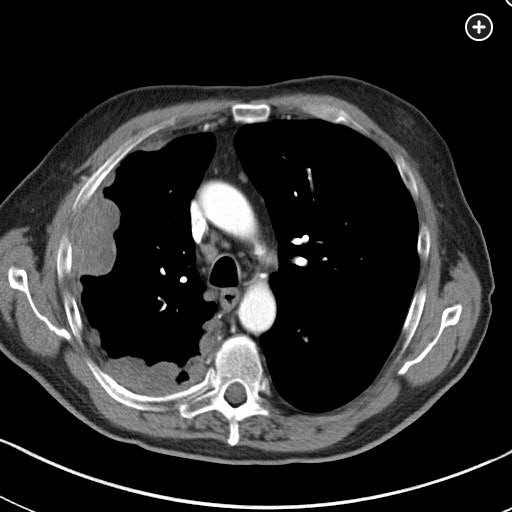
Pleural mesothelioma forms on the mesothelial surfaces of the thoracic cavity, but these malignancies may also develop on the peritoneum, the pericardium, or even the tunica vaginalis surrounding the testes. This neoplasm has been reported as presenting most commonly between the ages of 50 and 75, and its incidence is gradually declining in the United States because of regulatory controls, cessation of production of asbestos-related products, and improved personal protective equipment. Occupations such as labor-associated roles in the shipping industry, maintenance and production of boilers and heating plants, and automobile mechanics with exposure to brakes with asbestos linings have been noted to a have greatest prevalence of mesothelioma. Most patients complain of a nonproductive cough and may have dyspnea. A unique feature to mesothelioma is development of diffuse chest wall pain that may become more focal over time. In addition, palpable subcutaneous masses have been reported. Pleural effusions are common and are right sided 60% of the time. Five percent may present with bilateral effusions. Pleural plaques are also common, and one out of five patients develops bibasilar fibrosis, characteristic of chronic asbestosis. CT scanning may show pleural-based nodular abnormalities. PET scanning may be considered diagnostically, since mesothelioma does have hypermetabolic characteristics.
MPM is a rare insidious malignancy in both men and women that usually manifests with advanced disease (choice D is incorrect). The incidence of MPM is generally higher in male than female patients and is attributed to historical differences in exposures with world-standardized incidence rates per 100,000 persons of 0.7 and 0.3 in the United States and 1.7 and 0.4 in Europe (for male and female patients, respectively). The incidence is highest in countries with the greatest previous asbestos use such as the Netherlands, United Kingdom, and Australia. From a global perspective, the five most common cancers in women are breast, colorectal, lung, cervix uteri, and thyroid. MPM occupies position 30 among all the cancers in women and is considered rare.
MPM is a disease of the elderly, being rare in those younger than 50 years, with a sharp rise in incidence thereafter and a median age at diagnosis of 76 years. The predominant cause is inhalational exposure to asbestos. There is a very prolonged latency period between exposure to asbestos and the development of MPM. Exposure to ionizing radiation to supradiaphragmatic fields in the treatment of malignancy (Hodgkin lymphoma, non-Hodgkin lymphoma, testicular cancer) has also been associated with a statistically significant increase in the risk of MPM, although this accounts for only a small fraction of MPM cases. A genetic predisposition for MPM has been identified (mutation in the BAP1 gene) that has been associated with other cancers, especially ocular melanoma. The majority of patients present with a gradual onset of nonspecific symptoms, such as chest pain, dyspnea, cough, hoarseness, or dysphagia, which occur in the setting of extensive intrathoracic disease. Chest imaging, particularly with chest CT scanning, typically shows unilateral pleural thickening greater than 1 cm (nodular pleura or concentric pleural thickening) and pleural effusion with involvement of the mediastinal pleura surface like the one observed in this patient.
Imaging
Chest x-ray will show pleural thickening and possibly pleural effusion.
Aggressive therapy including combined pneumonectomy, chemotherapy and radiation has been shown to improve outcomes of patients with epithelioid type mesothelioma.
Histology
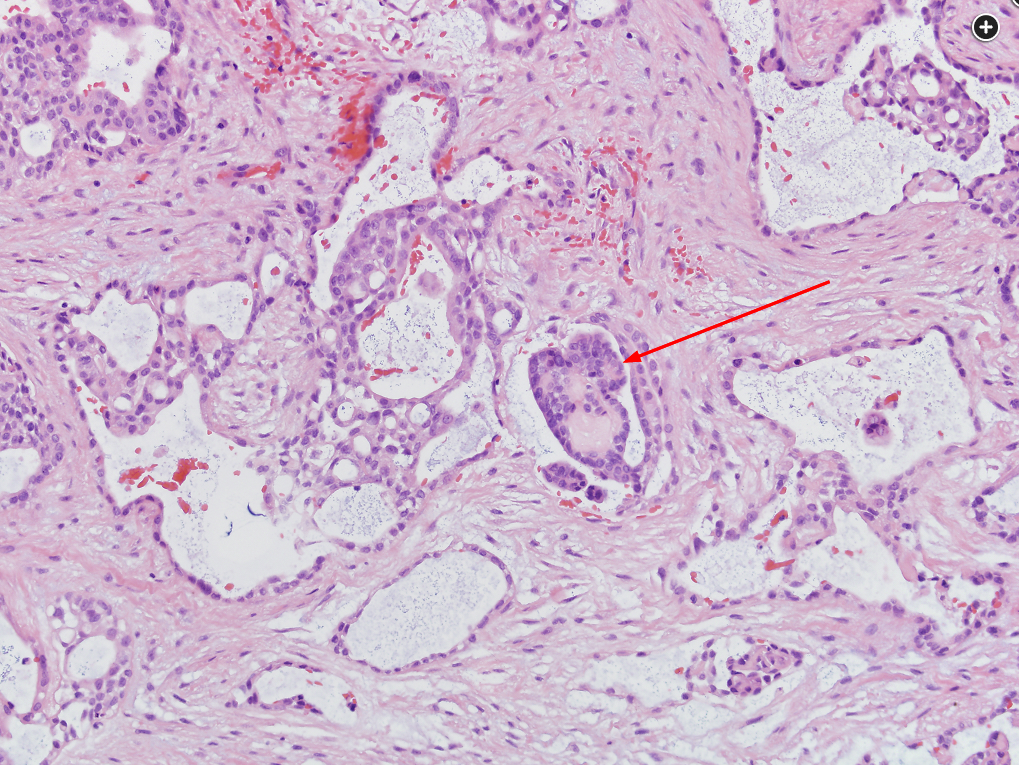
Malignant mesothelioma is much less common than bronchogenic carcinoma, and it requires significant diagnostic acumen by the pathologist and clinician to differentiate it from adenocarcinoma of the lung. Adequate tissue sampling is required, and a combination of recognizing histologic features and use of immunohistochemical staining techniques helps to confirm the diagnosis. The photomicrograph shown in Figure 3 reveals the presence of an epithelial mesothelioma. There are glandlike spaces, some associated with papillary structures (indicated by the red arrow). Among the three main variants of mesothelioma (epithelioid, sarcomatoid, and biphasic), epithelial mesotheliomas have the most favorable prognosis. Sarcomatoid variations portend a less favorable outcome. Mortality rates for all forms of malignant mesothelioma are high. Immunohistochemical staining and molecular testing help to confirm a diagnosis.
The diagnosis of MPM is established by morphologic and immunohistochemical features of a cytologic or surgical specimen. Nearly all MPMs are diffuse, although very rarely tumors are localized, defined by a single circumscribed mass with no clinical or histological evidence of spread. There are three histological subtypes: epithelioid, sarcomatoid, and biphasic. Epithelioid MPM can usually be diagnosed by using a combination of two MPM-associated markers (eg, calretinin, Wilms tumor antigen-1, cytokeratin 5/6) and two (adeno)carcinoma-associated markers (eg, carcinoembryonic antigen [CEA], Ber-EP4, MOC-31), supplemented by other markers dependent on the possibility of known, suspected, or occult malignancies (Figure 4).
In patients with MPM, several biomarkers are selectively elevated, including soluble mesothelin-related peptides, fibulin-3, and osteopontin, although they are not routinely used in the diagnosis of MPM (choice C is incorrect). The differential diagnosis includes benign conditions such as inflammatory reactions, as well as malignant processes, including metastasis from other solid tumors and lung adenocarcinoma. MPM has a poor prognosis with an overall survival of 9 to 17 months.
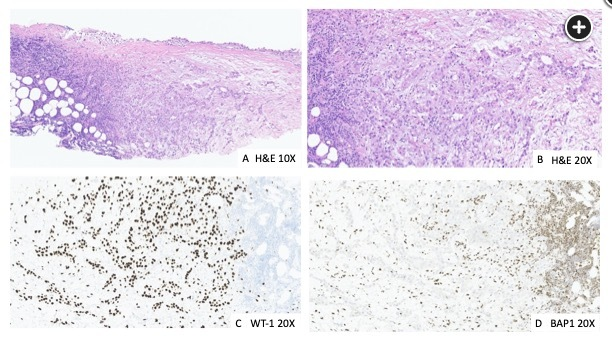
Part A shows a hematoxylin and eosin (H&E) stain intermediate-power field revealing epithelial proliferation that invades the full thickness of the pleura and into fat. Part B is an H&E higher-power field that shows epithelial proliferation forming mostly tubules. The epithelial cells are mildly to moderately atypical. Part C is a Wilms tumor 1 (WT1) immunohistochemistry stain showing a strong and diffuse nuclear staining. WT1 is one of the many mesothelial stains used. This tumor was also positive for calretinin and D2-40. Part D is a BAP1 immunohistochemistry stain that is lost (ie, the nuclei of the tumor cells are not staining in contrast to the lymphocytes, which retain the normal nuclear staining of BAP1).1
Pleural plaques are not necessarily a marker for developing lung cancer, but individuals with pleural or diaphragmatic plaques identified on CT imaging who also had lung cancers end up having higher mortality rates (choice D is correct). A retrospective review of more than 2,300 Italian workers undergoing screening spiral low-dose CT (LDCT) found a higher frequency of lung cancer among subjects confirmed to have pleural plaques (9.7% vs 4.2%), whether they were aware of prior asbestos exposure or not, and a hazard ratio of 5.48 (95% CI, 1.61-18.70) was calculated for mortality from lung cancer for those with pleural plaques.
According to World Health Organization data, at least 125 million workers have been exposed to asbestos in the workplace across the globe, with more than 100,000 individuals dying each year from asbestos-related diseases due to occupational exposure. Although asbestiform fiber production is banned in 52 countries, significant amounts of the product continue to be used within Africa, Latin America, Eastern Europe, and Asia, with China being the largest consumer of asbestos worldwide. Even in nations where asbestos has been outlawed, exposures continue to be recorded related to mitigation and removal of asbestos-related products.2
Links to this note
-
pulmonary diseases that are less common among smokers than nonsmokers
- Cigarette smoking does not increase risk for mesothelioma.[^1]
-
mesothelioma is associated with BAP1 mutation
- related: mesothelioma
-
mesothelioma vs adeno staining
- related: mesothelioma