primary graft dysfunction following lung transplant
- related: Pulmonology
- tags: #literature #pulmonology
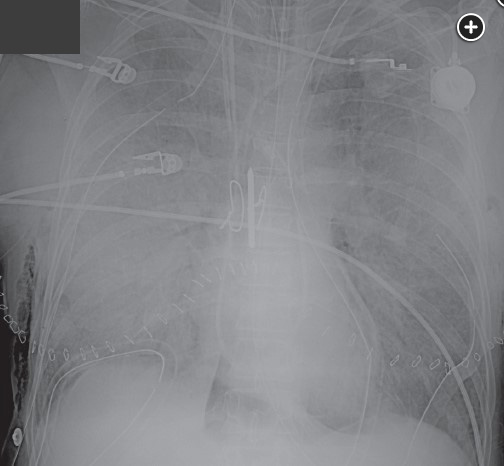
This patient has developed primary graft dysfunction (PGD) following lung transplantation (LT). Long-term, PGD has been shown to be associated with an increased risk of development of the bronchiolitis obliterans syndrome phenotype of chronic lung allograft dysfunction (CLAD). PGD is likely caused by ischemia-reperfusion injury to the lung. It is a diagnosis of exclusion made clinically in the appropriate postoperative setting—usually within the first 72 hours after transplantation—and is characterized by decreased gas exchange, a reduction in lung compliance, and the appearance of new alveolar infiltrates. PGD was the subject of a four-part consensus statement discussing definition, causes, outcomes, risk factors, treatment, and prevention. There is a PGD Severity Grading system based on the impairment in gas exchange (based on the Pao2/Fio2 ratio) and the presence of infiltrates and is graded 0-3. Grade 0 is the absence of pulmonary edema on chest radiograph and Pao2/Fio2 >300, grades 1-3 all have pulmonary edema on chest radiograph and Pao2/Fio2 of >300, 200 to 300, and <200, respectively. The incidence of PGD is about 30% for all grades combined and 15% to 20% for grade 3 PGD at 48 to 72 hours. PGD is assessed at the time of reperfusion of the graft, and at times 24, 48, and 72 hours following LT. The patient described in this case has PGD grade 3. Diffuse alveolar damage is the characteristic pathology, although it would be unusual to obtain pathology unless during autopsy.
Risk factors for PGD have been defined and are based on donor, recipient, and operative factors and include donor smoking and probably donor aspiration, chest trauma/contusion, alcohol use, and undersized donor graft relative to recipient. Operative risk factors include need for cardiopulmonary bypass, large volume intraoperative transfusions, delayed chest closure, prolonged ischemic times and increasing reperfusion Fio2. Recipient risk factors may include abnormal body weight, moderate to severe pulmonary hypertension, and a pretransplant diagnosis other than COPD or cystic fibrosis.
The shortand long-term outcomes of PGD correlate with scores at 48 and 72 hours, and the higher grade (PGD 3) and longer duration at 48 to 72 hours of PGD appear to have the worse prognosis and greatest impact on long-term outcomes. In the short term, PGD is associated with increased duration of mechanical ventilation, ICU, and hospital length of stay, hospital resource utilization, and higher costs. Long-term, PGD is associated with an increased risk of development of the bronchiolitis obliterans syndrome phenotype of CLAD, and with higher grades and longer duration, both 90-day and 1-year mortality (choice A is incorrect). A large multicenter prospective, cohort study in patients with severe PGD at 48 or 72 hours found an unadjusted 90-day mortality rate of 23% vs 5% for those without severe PGD. In another study, 90-day death rates for different grades of PGD were: grade 1= 7%, grade 2 = 12%, and grade 3 = 33%, respectively. Several studies have shown that grade 3 PGD was associated with a greater risk of 1-year all-cause mortality compared with the group without grade 3 PGD. In one study, all-cause mortality at 1 year was 64.9% with PGD 3 >48 hours vs 20.4% in the non-PGD/non-PGD 3 group. In another large study, patients with severe PGD had an increased 90-day mortality (absolute risk of 18%) and an increased 1-year mortality (absolute risk of 23%). There is no clear increase in subsequent acute cellular rejection or infection following PGD. There may be an increased risk of acute humoral or antibody mediated rejection.
Management of PGD includes supportive care with low tidal volume ventilation, empiric antibiotics, and sometimes inhaled nitric oxide (NO), inhaled prostaglandin, and ECMO (ideally started within 24 hours of the development of severe PGD). Retransplantation is associated with poor outcome and is avoided.
PGD is a diagnosis of exclusion and other conditions in the differential diagnosis include volume overload (less likely in this patient with a normal pulmonary artery catheter occlusion pressure), the rare occurrence of hyperacute rejection, infection, antibody mediated rejection, myocardial dysfunction, pneumonia, pulmonary thromboembolism, and venous anastomotic stenosis that can be evaluated with a transesophageal echocardiogram.1