treat large vessel occlusion with endovascular thrombectomy
- related: Neurology
- tags: #literature #neuro
The question of endovascular intervention for large vessel occlusions (LVOs) of the cerebral circulation is an active one. Particular attention has been focused on the time that has elapsed since onset of symptoms, the volume of the affected cerebral tissue, and the proportion of ischemic vs infarcted tissue. As the duration of time from onset of symptoms to therapy increases, the success of systemic thrombolysis decreases. The time window with the highest probability of success for systemic thrombolysis is 4.5 h, with some studies suggesting possible, albeit diminished, efficacy up to 6 h. Systemic thrombolysis after 6 h (as in this patient’s case) is generally not recommended, on the basis of disappointing results from clinical trials.
Endovascular therapies, including directed infusion of thrombolytics, suction, and fragmentation devices, have also been used in the treatment of LVO (typically within 24 h of the time the patient was last known to be well). Previously, these therapies were recommended for LVO with smaller infarcts, given a concern for an increased risk of secondary bleeding in larger infarcts after reperfusion. Recent studies, though, have demonstrated the efficacy of these endovascular therapies for patients with LVO and large ischemic regions. The SELECT2 (N = 352 patients) and RESCUE-Japan LIMIT (N = 203) trials both investigated this question among adults with large-volume stroke (generally an internal carotid or main-trunk middle cerebral artery occlusion with an ischemic volume consistent with a large stroke). In both studies, functional outcomes (at 90 days) were superior among patients treated with endovascular thrombectomy than among those treated with medical therapy alone. The improvements in functional outcomes appeared sufficient to justify the risk of procedural complications.
SELECT2 enrolled patients with a large infarct (Alberta stroke program early CT score [ASPECTS] of 3-5 or >50-mL volume) within 24 h of symptom onset, and RESCUE-Japan LIMIT enrolled patients with similar infarcts. The ASPECTS scale ranges from 0 to 10, with lower scores indicating larger volumes of ischemic tissue on initial noncontrast-enhanced CT scans. In both trials, the primary efficacy end point was the well-validated stroke outcome measure, the Rankin Scale, at 90 days, with minor differences in the method of analysis. In the RESCUE-Japan LIMIT trial, 31% vs 13% (intervention vs control) of patients achieved a favorable outcome (independent ambulation or better) on the Rankin Scale; in the SELECT2 trial, 38% vs 19% (intervention vs control) did. Although the SELECT2 trial closed on the basis of an unplanned interim analysis precipitated by publication of the results of the RESCUE-Japan LIMIT trial, the findings from both trials appear secure.
There is no evidence that using half-dose systemic thrombolytics (occasionally proposed for the treatment of pulmonary embolism) is appropriate for patients with an acute ischemic stroke.
Although dual antiplatelet therapy has been studied in a variety of atherosclerotic conditions, there is no available evidence to suggest that dual antiplatelet therapy is an efficacious therapy in this setting.
Although decompressive hemicraniectomy is often used to treat cerebral edema that may threaten brainstem herniation, in this case the patient is alert, and the only edema is adjacent to the area of ischemia or infarction. Although the patient in this case may ultimately progress to life-threatening cerebral edema, it is not appropriate at the present time to perform hemicraniectomy.123
This patient is a candidate for interventional thrombectomy if her core infarct is small and she shows evidence of a large vessel occlusion (LVO). This analysis can be accomplished with CT angiography and either CT or MRI perfusion scanning (choice C is correct). The last 10 years have seen a revolution in the treatment of acute ischemic stroke. Previous to four landmark studies, the major limitations of treatment of patients with acute ischemic stroke were the use of concomitant anticoagulation medications and the time last known well (LKW). The use of IV tPA is limited to 4.5 h (3 h in patients over the age of 80) from the last time they were seen or remember being without neurological symptoms.
Although the time window for tPA has not changed (choice A is incorrect), the timing of intravascular interventions has expanded, opening up the window of treatment for selected patients to as long as 24 h, which suggests that transitioning to management of stroke and secondary prevention is premature (choice B is incorrect). The initial landmark trials, MR CLEAN and SWIFT-PRIME, demonstrated the efficacy of mechanical evacuation of thrombus within 6 h of LKW in patients based only on demonstration of LVO. Additionally, because the bleeding risk is less with endovascular interventions than with tPA, patients on anticoagulation were included in the trial.
Although this was a big incremental advance, it still left a significant population of patients who wake up with symptoms without treatment options. In 2018, two trials addressed this issue directly. The DAWN trial and the DEFUSE 3 trial tested patients who present with stroke symptoms between 6 and 24 h (DEFUSE limited enrollment to less than 16 h) since LKW, provided there was evidence of a LVO by CT arteriography and evidence of a large perfusion/injury mismatch by either CT perfusion scan or MRI perfusion scan. Intervention produced better functional recovery to independence. The magnitude of the benefit can be inferred by determining the number needed to treat (how many patients need to be treated to expect one good outcome). In these trials, the number needed to treat was only three; three patients need treatment to ensure a good outcome in one. It is important to note that in both of these studies, perfusion/injury mismatch was estimated using RAPID computer software to limit therapy to patients who are likely to benefit. This stratification is seen as critical to making appropriate patient selection. Previous registries of patients treated with intravascular thrombolysis after 6 h without stratification had an increased risk of hemorrhage and showed no evidence of benefit compared with historical control. Diagnostic digital subtraction angiography has the advantage of transitioning to intervention more quickly and could show similar findings but is more cumbersome to perform than CT angiogram and perfusion imaging. Bringing on board the interventional team to perform a diagnostic angiography is resource intensive unless the pretest probability of intervention is high, which CT angiogram and perfusion imaging offers (choice D is incorrect).
These studies have changed clinical practice quickly, leading to an increased number of patients taken to intervention. This has also led to the development of screening scales that can be used in EDs and hospitals to determine which patients are likely to have LVO. A number of scales have been developed, including the VAN (vision, aphasia, neglect) and LARIO (large artery intracranial occlusion) scales. Most have been developed to be used in EDs to help triage patients. These scales have been adopted quickly in clinical practice, but because they are new there have been no head-to-head comparisons.45678
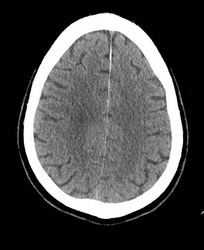
A 65-year-old woman with a past medical history of hypothyroidism and depression presented to an outside hospital with a profound right hemiparesis, right facial droop, aphasia, and right hemineglect. She was last seen well at 11 PM the previous evening and was therefore not considered eligible for IV tissue plasminogen activator (tPA). A representative image from her noncontrast head CT scan on admission is shown in Figure 1. She is now transferred to your Joint Commission–certified comprehensive stroke center for further care, arriving at 10 AM. What is the next appropriate intervention?
- CTA/perfusion study
Links to this note
Footnotes
-
Sarraj A, Hassan AE, Abraham MG, et al; SELECT2 Investigators. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388(14):1259-1271. PubMed ↩
-
Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386(14):1303-1313. PubMed ↩
-
Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. PubMed ↩
-
Goyal M, Menon BK, van Zwam WH, et al; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. PubMed ↩
-
Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. PubMed ↩
-
Teleb MS, Ver Hage A, Carter J, et al. Stroke vision, aphasia, neglect (VAN) assessment-a novel emergent large vessel occlusion screening tool: pilot study and comparison with current clinical severity indices. J Neurointerv Surg. 2017;9(2):122-126. PubMed ↩
-
Vidale S, Arnaboldi M, Frangi L, et al. The large artery intracranial occlusion stroke scale: a new tool with high accuracy in predicting large vessel occlusion. Front Neurol. 2019;10:130. PubMed ↩