use ascorbic acid to treat methemoglobinemia in G6PD deficient patients
- related: methemoglobinemia has reduced O2 saturation with normal PaO2
- tags: #literature #pulmonology #icu
This patient has classic features of methemoglobinemia, including SpO2 in the mid-80% range despite supplemental oxygen, along with blood that has a dark chocolate brown appearance (Figure 1) despite a PaO2 of 280 mm Hg. A high oxygen saturation gap between calculated arterial oxygen saturation of 99% and SpO2 of 84% is noted. In this case, the best intervention of those offered is administration of ascorbic acid IV (choice C is correct) and not methylene blue IV (choice D is incorrect).
Acquired and congenital forms of methemoglobinemia have been described with medications as the key precipitants of acquired methemoglobinemia (Figure 2). In this case, the precipitating agent was rasburicase, a recombinant form of urate oxidase that rapidly converts uric acid to allantoin but also generates hydrogen peroxide, which oxidizes the heme moiety of hemoglobin from ferrous (Fe 2+) to ferric (Fe 3+) state and thus creates methemoglobin. This occurs only if reduced nicotinamide adenine dinucleotide phosphate (NADPH) is not present. Importantly, conversion of NADP to NADPH depends on glucose-6-phosphate dehydrogenase (G6PD). Although the patient’s actual G6PD level is not available at the time of treatment decision, this patient is of Mediterranean descent, which places him at high risk of having G6PD deficiency, similar to individuals of Puerto Rican, African, or Southeast Asian descent. Additionally, the majority of reported cases of rasburicase-induced methemoglobinemia occur in the setting of G6PD deficiency, predisposing affected people to hemolysis and methemoglobinemia. The presence of strongly suspected G6PD deficiency has important treatment ramifications. The traditional treatment for methemoglobinemia is methylene blue IV, which reduces ferric iron to ferrous iron via an NADPH-dependent process. However, in patients with G6PD deficiency (and thus low NADPH), methylene blue is ineffective and can promote further hemolysis and worsen methemoglobinemia (choice D is incorrect).
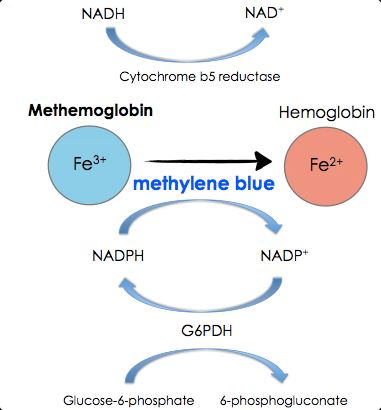
The antioxidants ascorbic acid and N-acetylcysteine act as electron donors independent of NADPH and can lower methemoglobin levels. Ascorbic acid is considered the treatment of choice when symptomatic methemoglobinemia occurs in the setting of proven or strongly suspected G6PD deficiency (choice C is correct).
The optimal dose is unknown, but 1 to 2 mg/kg is commonly used, with improvement typically occurring rapidly. A repeat dose can be administered if symptoms do not improve in 30 to 60 min. Methylene blue may precipitate hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency, as well as serotonin syndrome in individuals taking serotonergic agents, so it should be avoided in these cases. Some reports suggest the use of ascorbic acid as an alternative therapy in these situations, although it is substantially slower acting than methylene blue and the optimal regimen is unknown.
One might argue that ascorbic acid should be the first treatment of methemoglobinemia in TLS, given the high likelihood of rasburicase causation and G6PD deficiency. Finally, red cell transfusion or even exchange transfusion can be used in selected cases. An individualized approach to treatment of methemoglobinemia includes consideration of the severity of the symptoms, the methemoglobin level, underlying severe cardiopulmonary disease, and presence of G6PD deficiency. Use of the offending agent is discontinued in all cases. In this patient’s case, symptoms are minimal, cooximetry results are pending, and although the G6PD level is pending, G6PD deficiency is very likely. Methemoglobin levels <20% are generally well tolerated, with minimal symptoms, and can be treated conservatively. Patients with methemoglobin 20% to 50% often have progressive cardiopulmonary and neurologic manifestations and generally require treatment. Some experts select as a threshold for treatment a methemoglobin level >30% or significant symptoms. Methemoglobin levels >50% generally have severe manifestations such as coma, seizures, arrhythmias, and lactic acidosis and require aggressive management. Death is common, with rates exceeding 70%.1234
Links to this note
Footnotes
-
Ibrahim U, Saqib A, Mohammad F, Atallah JP, Odaimi M. Rasburicase-induced methemoglobinemia: the eyes do not see what the mind does not know. J Oncol Pharm Pract. 2018;24(4):309-313. PubMed ↩
-
Jung S, Sayad K, Staitieh BS. Low hemoglobin saturation in the setting of hyperuricemia. Ann Am Thorac Soc. 2019;16(11):1447-1450. PubMed ↩
-
Marinacci LX, Simeone FJ, Lundquist AL, Kuter DJ, Mahowald GK. Case 38-2020: a 52-year-old man with cancer and acute hypoxemia. N Engl J Med. 2020;383(24):2372-2383. PubMed ↩