acute leukemia and hyperkeukocytosis
- related: Oncology
- tags: #literature #hemeonc
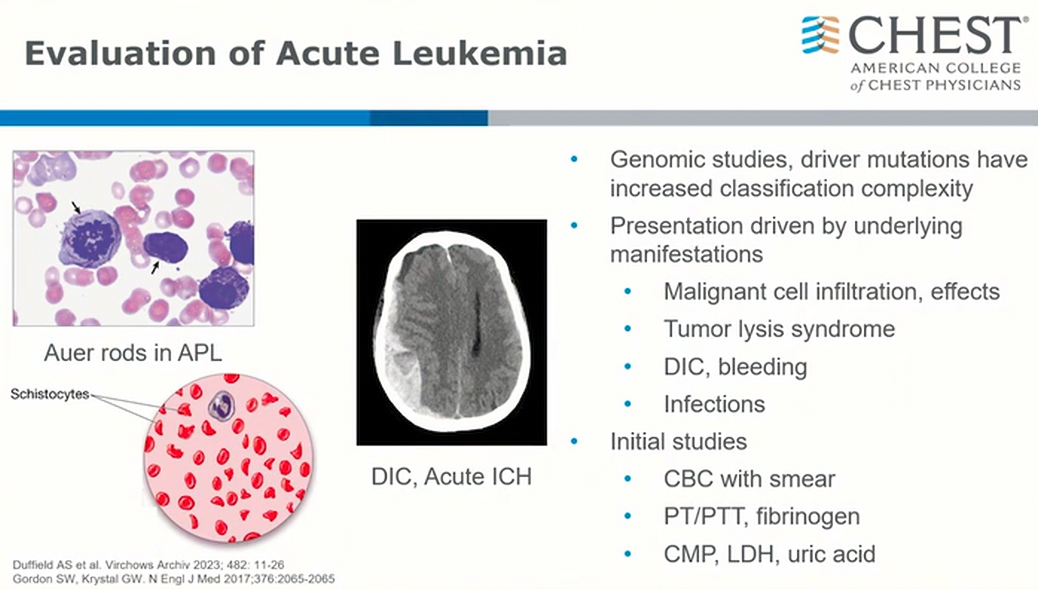
- include hyperleukocytosis in presentation
- schistocytes: DIC
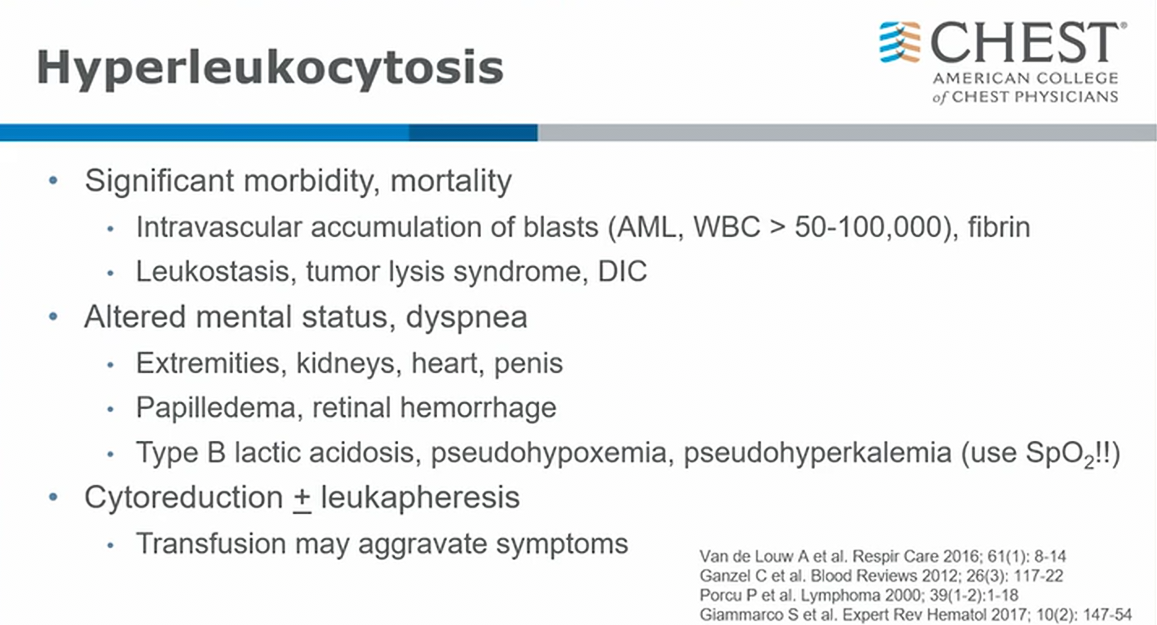
- aka leukostasis
- blasts > 50 with symptoms
-
80 very concerning
-
100 ppx leukopheresis
- develops from acute leukemia, problem from too much blasts
- brain and lungs most commonly involved
- pseudohypoxemia and pseudohyperkalemia: so many cells in blood samples drawn up that gobble up all oxygen = artificially low PaO2 than SpO2 on monitor. Lysis in the sample leads to artificially high K. (very high WBC can cause false hypoxemia leukocyte larceny)
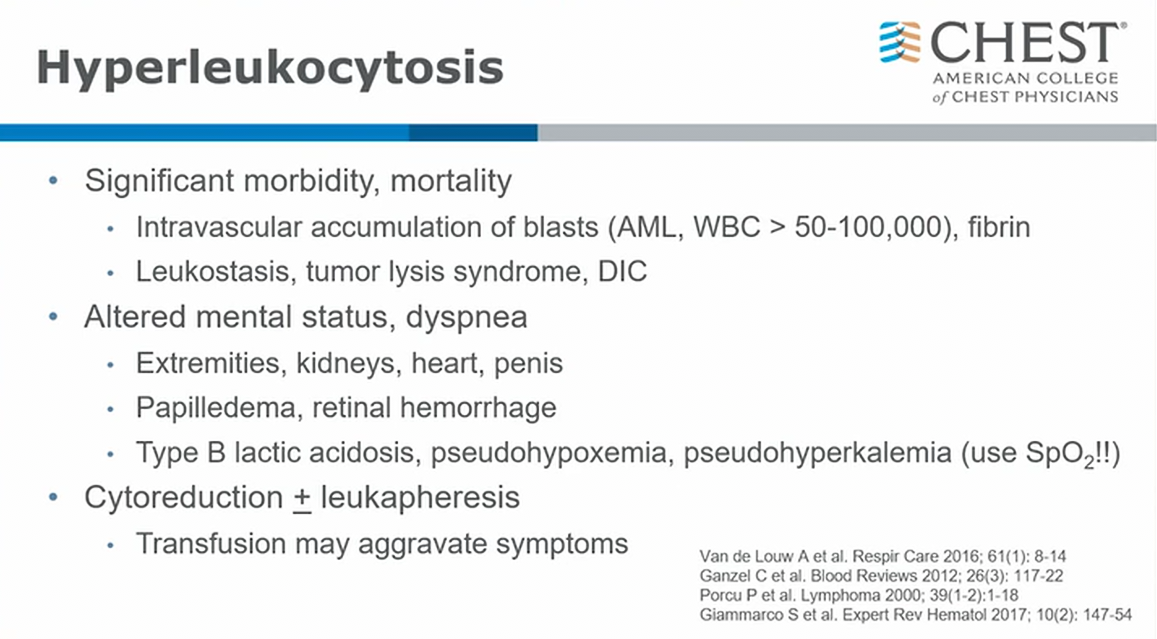
- recent years shift in management
- cytoreduction is core (treatment of underlying malignancy)
- leukapheresis: actually mixed data on efficacy. In general practice moved away from leukaphresis1
This patient does not meet criteria for hyperleukocytosis, which is a total leukemic blood cell count of 50,000-100,000/μL (50-100 × 109/L), most typically seen in patients with hematologic malignancies such as acute myeloid leukemia (AML) or chronic myeloid leukemia (CML). Patients with CML rarely develop symptoms of leukostasis in the chronic phase, but it can be seen in myeloid blast crisis with very high blast counts. Leukostasis can occur when WBCs coalesce in the microvasculature, and most commonly presents with respiratory distress and/or neurological symptoms. It is characterized by a high blast cell count and evidence of decreased tissue perfusion. Pulmonary signs and symptoms include dyspnea and hypoxia, with variable interstitial or alveolar infiltrates on chest radiography. A hallmark is decreased PO2 on arterial blood gas measurement due to oxygen uptake by the large numbers of blast cells. Pulse oximetry should be used to assess SpO2. Neurological signs and symptoms include visual changes, headache, dizziness, tinnitus, gait instability, and decreased levels of consciousness. Patients with hyperleukocytosis have an increased risk of intracranial hemorrhage that persists for at least a week after the reduction of the WBC count, perhaps from a reperfusion injury. Leukapheresis is used as initial therapy for leukostasis, as rapid lowering of the WBC count is essential, but is not indicated in this case.
Leukemias manifesting with acute hyperleukocytosis are associated with a very high risk of early mortality, predominantly due to respiratory failure or intracranial bleeding. Close collaboration between intensivist and hematologist is often necessary in this emergency setting, underscoring the importance of familiarity with the common features of this condition. Spurious hypoxemia—an artifactual decrease in PaO2—is a unique finding that can be seen in these patients and is due to increased oxygen consumption by leukocytes present in the arterial blood gas sample even when it is placed on ice and rapidly processed. When there is a discrepancy in values, SpO2 may be more reliable than PaO2 in this setting (choice B is correct).
Hyperleukocytosis is defined as a total leukemia blood cell count greater than 50,000 to 100,000/μL (50 to 100 × 109/L). Leukostasis (also called symptomatic hyperleukocytosis) is a pathologic diagnosis characterized by microvascular WBC plugs. It is more typically diagnosed clinically, however, in patients presenting with hyperleukocytosis and symptoms of decreased tissue perfusion. Although hyperleukocytosis can be seen with acute myeloid, acute lymphoblastic, chronic lymphocytic, or chronic myeloid leukemia, the symptoms associated with this condition are most commonly seen with the large, poorly deformable blasts in acute myeloid leukemia (choice A is incorrect).
Monocytic and myelomonocytic acute myeloid leukemia subtypes (M4 and M5) and a variety of _FLT3-_ITD and MLL gene mutations on 11q23 have been identified as risk factors for hyperleukocytosis. Although the exact pathophysiologic mechanism for leukostasis remains poorly defined, increased blood viscosity and decreased blast deformability have been classically described. More recent studies have found that leukocyte adhesion to the endothelium is mediated by the interactions of selectins expressed on endothelial cells and leukocytes and by integrins and cell adhesion molecules on circulating blasts. When activated by the proinflammatory cytokines IL-1β and tumor necrosis factor-α, these interactions lead to slow rolling of leukocytes along the endothelium and migration into the interstitial space. Leukemic blast obstruction and increased metabolic demand in the microvasculature, cytokineand matrix-metalloproteinase-mediated endothelial damage, and blast extravasation have all been proposed as mechanisms for local tissue hypoxia and injury. Respiratory and neurologic symptoms due to lung and brain microvascular involvement are most common, and findings of myocardial, bowel, renal, and limb ischemia and priapism can also be seen. Fever is also common and can be due to inflammation from leukostasis or concurrent infection.
A number of other laboratory test result abnormalities can be seen with hyperleukocytosis. Circulating blast fragments may result in an overestimation of platelet count with automated blood cell counters, underlining the importance of peripheral smear confirmation in this setting. Use of heparinized tubes will reduce the risk of spurious elevations in potassium, which is released by blasts during the in vitro clotting process. Findings of disseminated intravascular coagulation and tumor lysis can be present on presentation and may worsen during initial treatment. Hyperleukocytosis is a hematologic emergency that requires urgent cytoreduction. Treatment options include mechanical removal using leukapheresis and pharmacologic cytoreduction with hydroxyurea or chemotherapy. Use of leukapheresis has become increasingly controversial, as two meta-analyses have failed to demonstrate improvements in survival or complication rates with this resource-intensive intervention (choice C is incorrect).
Although there are now a number of targeted molecular acute myeloid leukemia therapies, patients with hyperleukocytosis were excluded from these trials without prior mechanical cytoreduction owing to the risk of severe tumor lysis syndrome. Common initial chemotherapy includes 7 + 3 (cytarabine and idarubicin). Concern about worsening blood viscosity typically drives a conservative approach of RBC transfusion during initial treatment, although continued platelet support is important to reduce the risk of hemorrhage due to both thrombocytopenia and disseminated intravascular coagulation (choice D is incorrect).234567
A 59-year-old patient is transferred to the ICU by the rapid response team for further evaluation and management of acute encephalopathy requiring emergent intubation. The patient was admitted for evaluation and management of a leukemic transformation after presenting with several weeks of worsening shortness of breath and fatigue. The medical history is significant for myelodysplastic syndrome with continued transfusion dependence despite five cycles of azacitidine, with pending referral for bone marrow transplant evaluation. Bone marrow biopsy was performed, and the patient started receiving hydroxyurea pending the development of an induction chemotherapy plan.
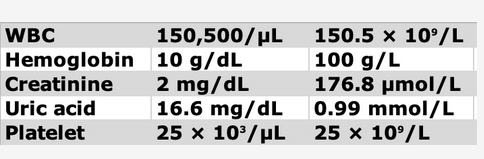
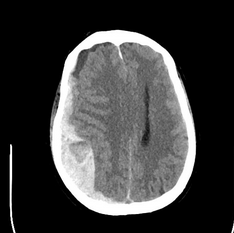
On ICU arrival, the temperature is 37.8°C, heart rate is 89/min, BP is 156/85 mm Hg, and respiratory rate is 25/min without spontaneous respiratory efforts with volume targeted assist-control ventilation. The patient is unresponsive without movement to painful stimuli, with dilated, minimally reactive pupils and no cranial nerve reflexes on rapid neurologic assessment. General examination demonstrates scattered crackles, a palpable spleen, and 1+ bilateral lower extremity edema. The WBC count is 150,500/μL (150.5 × 109/L) with 34% circulating blasts and multiple immature WBC and RBC forms. Laboratory test results are shown in Figure 1. The creatinine level is baseline normal. There is a mildly elevated lactate dehydrogenase level and unconjugated hyperbilirubinemia. Prothrombin and partial thromboplastin times are normal. Representative images of the chest CT scanning performed on admission and head CT scanning performed after intubation are shown (Figure 2 and Figure 3).
Which of the following statements regarding this clinical syndrome is correct?
Links to this note
Footnotes
-
Bewersdorf JP, Zeidan AM. Hyperleukocytosis and leukostasis in acute myeloid leukemia: can a better understanding of the underlying molecular pathophysiology lead to novel treatments? Cells. 2020;9(10):2310. PubMed ↩
-
Giammarco S, Chiusolo P, Piccirillo N, et al. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol. 2017;10(2):147-154. PubMed ↩
-
Porcu P, Cripe LD, Ng EW, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000;39(1-2):1-18. PubMed ↩
-
Shallis RM, Stahl M, Bewersdorf JP, et al. Leukocytapheresis for patients with acute myeloid leukemia presenting with hyperleukocytosis and leukostasis: a contemporary appraisal of outcomes and benefits. Expert Rev Hematol. 2020;13(5):489-499. PubMed ↩
-
Van de Louw A, Desai RJ, Schneider CW, et al. Hypoxemia during extreme hyperleukocytosis: how spurious? Respir Care. 2016;61(1):8-14. PubMed ↩