common toxic alcohols
- related: anion gap metabolic acidosis
- tags: #permanent
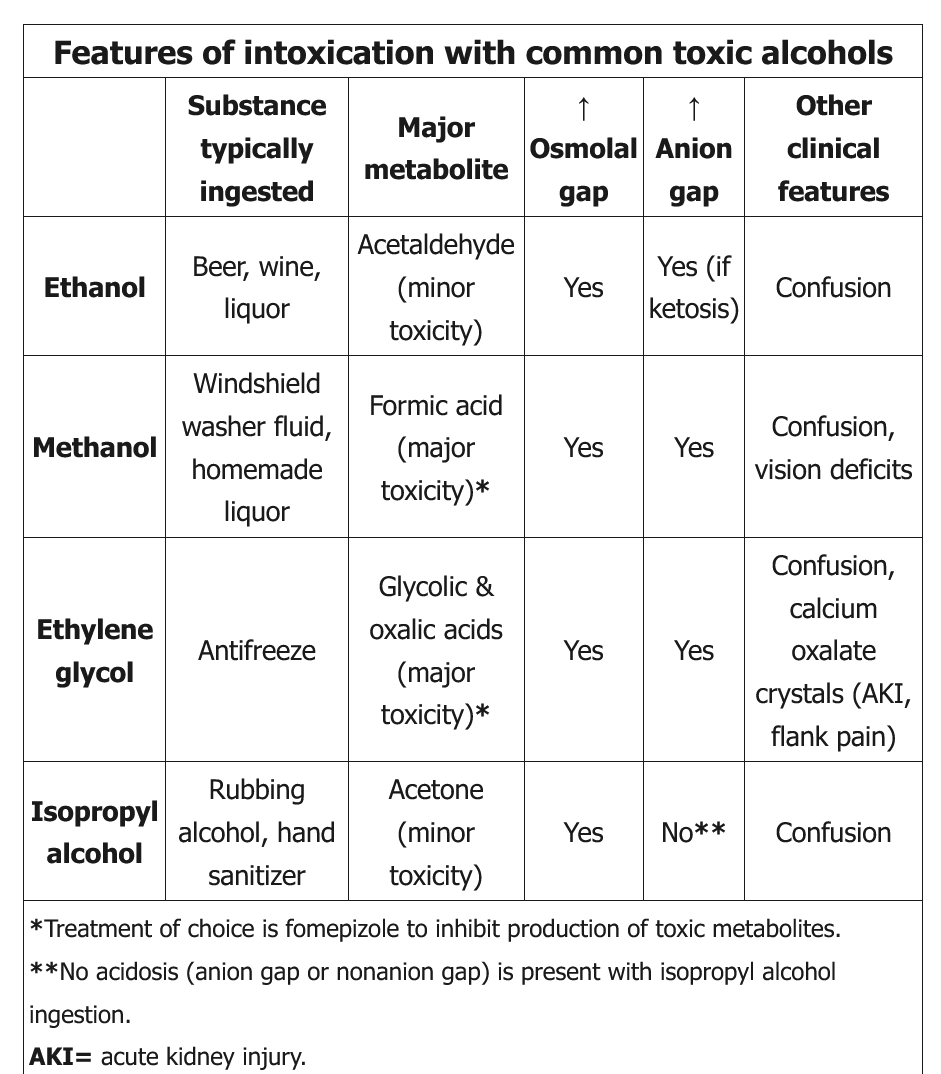
Ethanol
- Ethanol is the most common cause of an elevated osmolar gap; the contribution of ethanol to serum osmolality can be calculated by dividing the serum ethanol level by 4. Isolated ethanol ingestion causes only an elevated osmolar gap (without AGMA).
Ethylene Glycol
- laboratory confirmation of ethylene glycol intoxication may take days, empiric therapy with fomepizole and aggressive fluid resuscitation with crystalloids (250-500 mL/h intravenous initially) should be instituted in all cases to increase kidney clearance of the toxin and to limit deposition of oxalate in the renal cortex. Hemodialysis to clear the alcohol and toxic metabolites should be instituted in the context of any organ-specific toxicity (central nervous system depression, acute kidney injury, systemic collapse), severe acidemia, or very large ingestions.
Isopropyl alcohol
- Isopropyl alcohol, like ethanol, is a central nervous system depressant. It is rapidly metabolized by alcohol dehydrogenase to the ketone acetone, leading to elevated serum and urine ketone measurements. Some laboratories measure beta-hydroxybutyrate concentrations as a substitute for serum ketones, leading to a falsely low measurement in patients with isopropyl alcohol ingestion. Unlike methanol and ethylene glycol, isopropyl alcohol does not cause high AGMA and does not have toxic metabolites causing further damage (eg, blindness, renal failure). It does cause increased osmolar gap.
- Massive ingestions can cause respiratory depression and hypotension. Treatment is mainly supportive with intravenous fluids, pressors, and mechanical ventilation; hemodialysis can be initiated in severe cases. Fomepizole and gastrointestinal decontamination are usually not helpful.
Propylene glycol
- Propylene glycol, a solvent used to enhance the solubility of various intravenously administered medications, causes an increased anion gap metabolic acidosis through its acid metabolites, L-lactate and D-lactate. An increased osmolal gap accompanies the increased anion gap metabolic acidosis seen with propylene glycol. This patient’s clinical history and lack of lactic acidosis are not consistent with propylene glycol toxicity.
Methanol
- blurry vision, retinal edema, abdominal pain, photophobia
- formic acid build up in brain and cause optic swelling
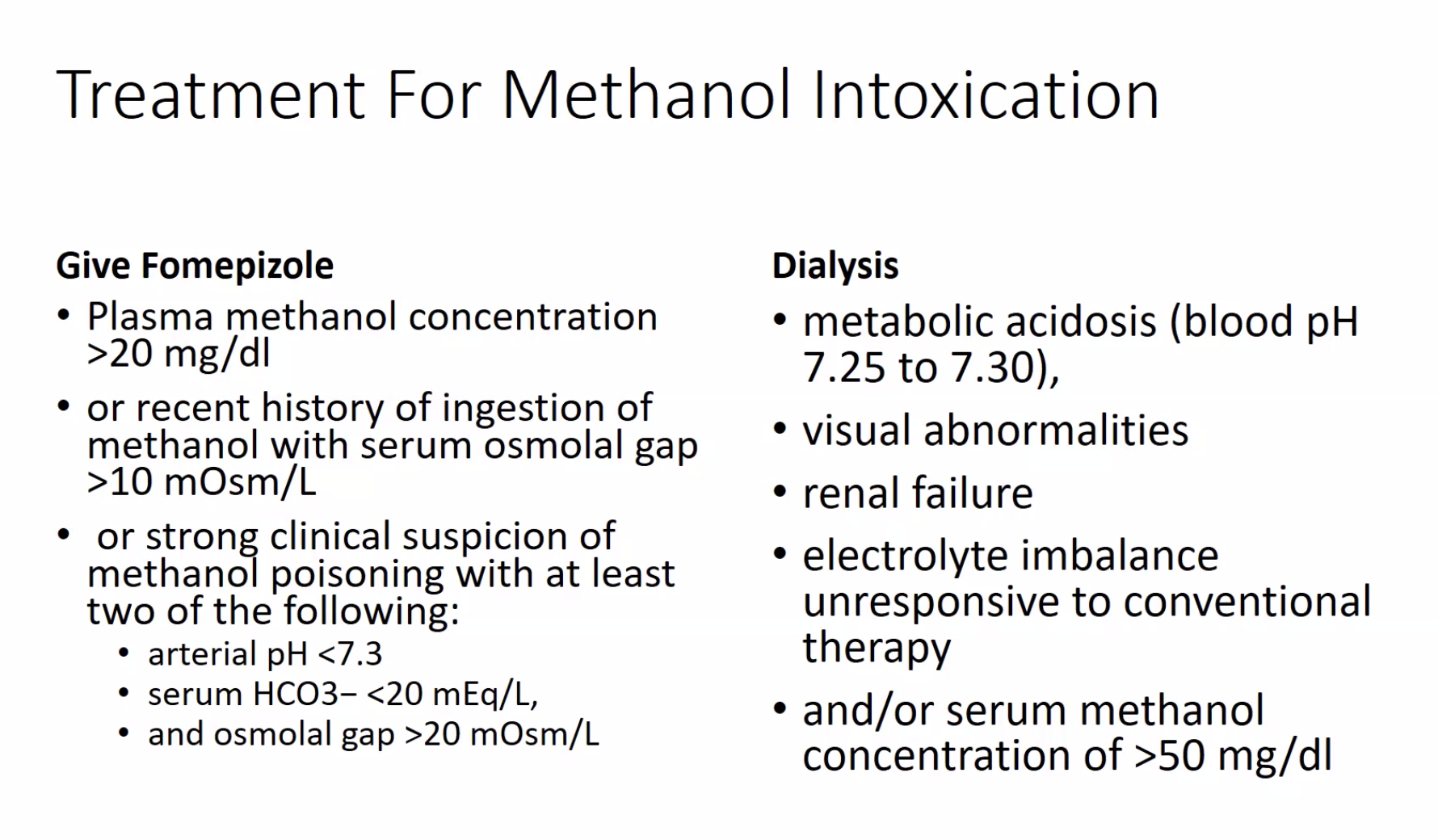
The toxicity of methanol and ethylene glycol arises not from the compounds themselves but primarily from highly toxic intermediate metabolites generated by the action of alcohol dehydrogenase (ADH), the key enzyme in their breakdown. In the case of methanol, ADH catalyzes the first oxidation of methanol to formaldehyde. Methanol is ultimately metabolized to formic acid, a potent organic acid that generates an anion gap metabolic acidosis and can also lead to blindness. The inhibition of ADH, therefore, becomes a crucial step in treatment. Fomepizole is a competitive inhibitor of ADH approved for the treatment of methanol poisoning in 2000, and should be administered in patients suspected of being poisoned. Ethanol is sometimes used as a competitive inhibitor of ADH, but it is not as effective as fomepizole, which this patient is already receiving (choice A is incorrect). Failure to clear acidosis rapidly after fomepizole treatment is an indication for dialysis. In the setting of methanol toxicity, the aim of dialysis is prompt elimination of methanol and its toxic metabolite, formic acid, while correcting metabolic acidosis. Duration of dialysis is based on methanol kinetics. Methanol is a small molecule of 32 Da, and zero protein binding, which allows for rapid elimination without a concern for postdialysis rebound effect, making hemodialysis ideal. The elimination half-life with hemodialysis of methanol is about 1.5 to 3.5 h (choice C is correct).
Continuous renal replacement therapy (CRRT) refers to any modality of dialysis that runs for 24 h with low blood or dialysate flow. The elimination half-life of methanol with CRRT is expected to be at least fivefold longer than with hemodialysis (choice D is incorrect). Sustained low-efficiency dialysis is a type of dialysis that runs only for up to 12 h a day; its efficiency is between that of a regular hemodialysis and CRRT.12345
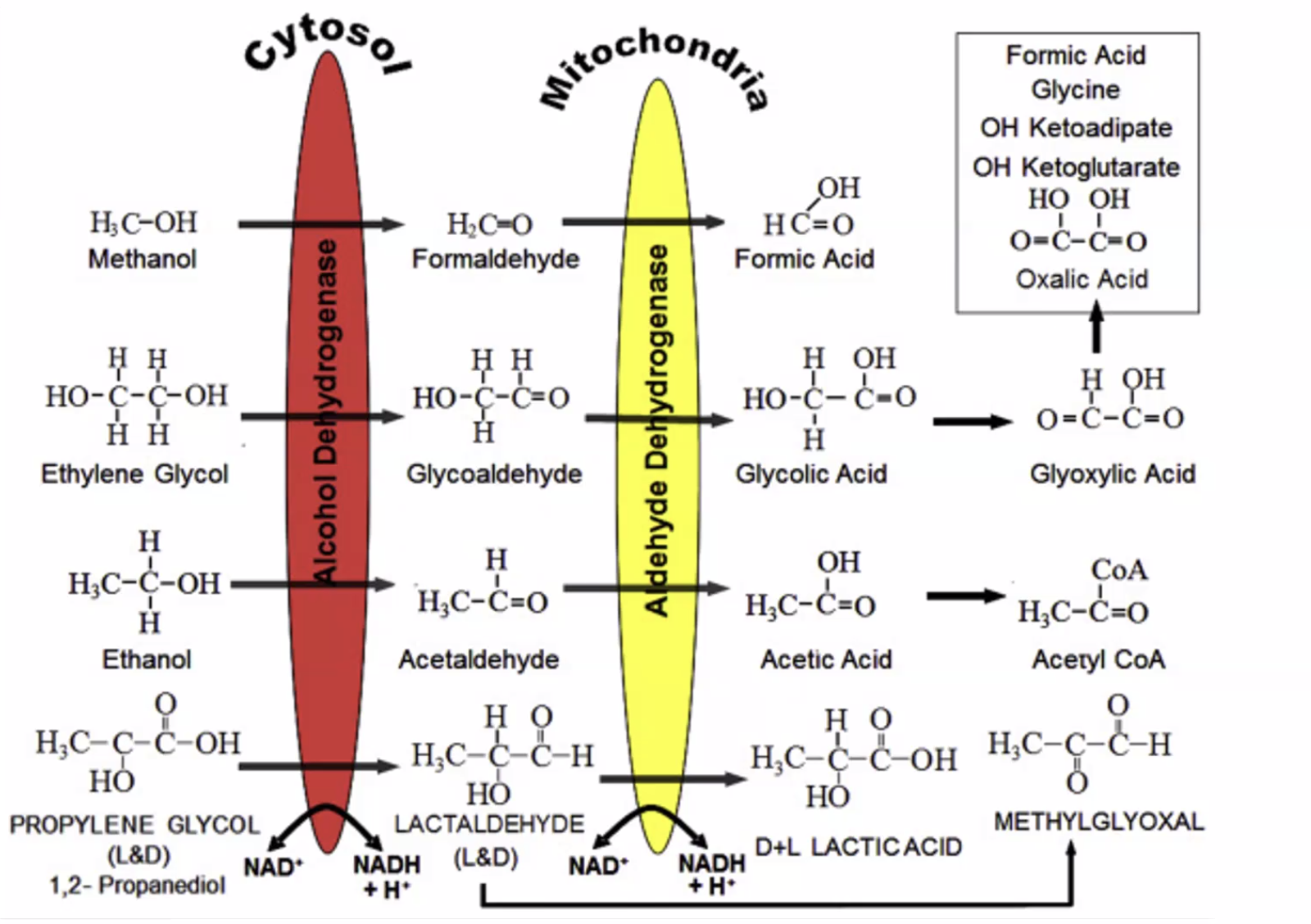
- ethanol does not have high AG because acetic acid is rapidly converted to acetyl coa
- isopropyl alcohol: osmolar gap but no AG acidosis. Rapidly changed to acetone
Links to this note
-
-
G: glycol: common toxic alcohols
-
M: methanol: common toxic alcohols
-
Footnotes
-
Giannini AJ, Miller N, Kocjan DK. Treating steroid abuse: a psychiatric perspective. Clin Pediatr (Phila). 1991;30(9):538-542. PubMed ↩
-
Kan G, Jenkins I, Rangan G, Woodroffe A, Rhodes H, Joyce D. Continuous haemodiafiltration compared with intermittent haemodialysis in the treatment of methanol poisoning. Nephrol Dial Transplant. 2003;18(12):2665-2667. PubMed ↩
-
Slaughter RJ, Mason RW, Beasley DM, et al. Isopropanol poisoning. Clin Toxicol (Phila). 2014;52(5):470-478. PubMed ↩
-
Zakharov S, Pelclova D, Navratil T, et al. Intermittent hemodialysis is superior to continuous veno-venous hemodialysis/hemodiafiltration to eliminate methanol and formate during treatment for methanol poisoning. Kidney Int. 2014;86(1):199-207. PubMed ↩