anion gap metabolic acidosis
- related: Nephrology, nonanion gap metabolic acidosis NAGMA causes
- tags: #nephrology #literature
Calculation
- plasma osmolar gap calculation
- example calculation winters formula agma
- use delta delta gap to calculate third acid base disorder
GOLD MARK

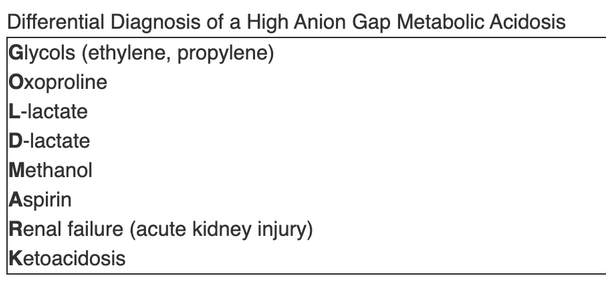
- G: glycol: common toxic alcohols
- O: oxoproline: pyroglutamic acidosis aka tylenol toxicity
- L: lactate lactic acidosis types
- D: D lactate
- M: methanol: common toxic alcohols
- A: ASA salicylate cause for AGMA
- R: renal failure, bicarb loss usually before stage 5 and regresses after stage 5. AG happens more in stage 5 when kidney is unable to excrete ammonia and other titratable acids.
- K: ketoacidosis
Links to this note
-
nonanion gap metabolic acidosis NAGMA causes
- related: Nephrology, anion gap metabolic acidosis
-
- related: anion gap metabolic acidosis
-
plasma osmolar gap calculation
- related: anion gap metabolic acidosis
-
DKA vs other types of ketoacidosis
- related: anion gap metabolic acidosis
-
example calculation winters formula agma
- related: anion gap metabolic acidosis
-
- related: anion gap metabolic acidosis